From cold fusion to pharmaceuticals
Published in Chemistry

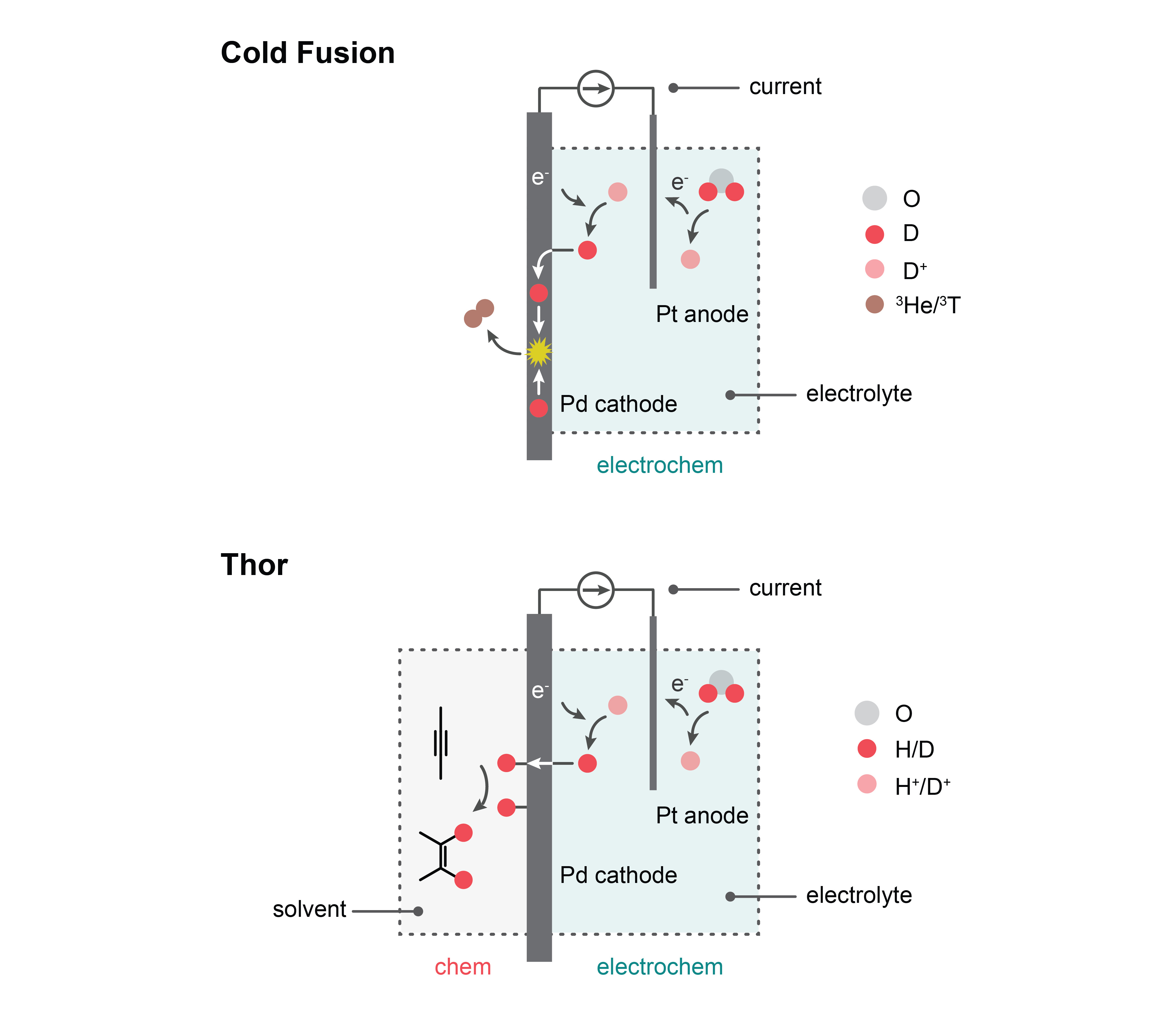
In 2015, Google invited us to revisit “cold fusion”, the 30-year old claim that electrolyzing heavy water at a palladium cathode could mediate nuclear fusion at meaningful rates. The notion of generating a nuclear fusion event by passing current through a cathode immersed in an aqueous medium would have been extraordinary, but we, like many academic groups before us, were not able to reproduce these controversial claims. Notwithstanding, we remain excited to explore the overlap of materials science and nuclear physics in an effort to define how metal lattices can mediate fusion events at colder temperatures than conventional nuclear processes1.
These cold fusion studies ultimately drew our attention to the fact that palladium can play three roles: electrode, membrane, and catalyst. In 2018, we elaborated on these collective properties by inserting a solid palladium foil in between two reaction chambers: an electrochemical chamber filled with water, and a chemical reaction chamber containing an organic reagent2. By passing electricity through the electrochemical chamber, we could harvest protons from water that would ultimately get reduced at the palladium foil and then pass through the foil to react with an organic reagent. This separation of water electrolysis from hydrogenation chemistry enables clean electricity to drive efficient hydrogenation chemistry in organic media at high rates of product formation3. We named this membrane reactor “Thor”.
Our latest study brings our story full circle4: we discovered that Thor can perform deuteration chemistry using deuterons by immersing palladium foil in heavy water. We contend that this set up, inspired by an electrochemical architecture developed for a cold fusion experiment, is relevant to the pharmaceutical industry because deuterated drugs offer longer residence times in the human body over their congeners. Considering that the FDA approved the first deuterated drug in 2017, there may exist opportunities to use Thor to selectively deuterate drugs or drug precursors without expensive reagents or specialized catalysts. The reactor also enables the recycling of D2O without purification, a potentially significant driver in cost reductions.
While we set out looking for cold fusion, we have discovered new ways to use membrane reactors. This technology presents entirely new opportunities for using renewable electricity to make useful chemicals, including high value drugs, without ever using gaseous H2 or D2!
References:
- Berlinguette, C. P., Chiang, Y.-M., Munday, J. N., Schenkel, T., Fork, D. K., Koningstein, R. & Trevithick, M. D. Revisiting the cold case of cold fusion. Nature, 570, 45-51 (2019). DOI:10.1038/s41586-019-1256-6
- Sherbo, R. S., Delima, R. S., Chiykowski, V. A., MacLeod, B. P. & Berlinguette, C. P. Complete electron economy by pairing electrolysis with hydrogenation. Nat. Catal. 1, 501–507 (2018). DOI: 10.1038/s41929-018-0083-8
- Sherbo, R. S., Kurimoto, A., Brown, C. M. & Berlinguette, C. P. Efficient electrocatalytic hydrogenation with a palladium membrane reactor. J. Am. Chem. Soc. 141, 7815–7821 (2019). DOI: 10.1021/jacs.9b01442
- Kurimoto, A., Sherbo, R.S., Cao, Y., Loo, N. W. X., & Berlinguette, C. P. Electrolytic deuteration of unsaturated bonds without using D2.” Nat. Catal. (2020). DOI: 10.1038/s41929-020-0488-z
Follow the Topic
-
Nature Catalysis

This journal brings together researchers from across all chemistry and related fields, publishing work on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, incorporating both fundamental and applied studies.

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in