From Magnetotactic to Magnetized: New Hosts for Magnetosome Biosynthesis
Published in Microbiology
Bacteria with a Compass
In 1958, while routinely observing water samples from various aquatic environments, an Italian medical doctor Salvatore Bellini stumbled upon a remarkable discovery: some bacteria exhibited a peculiar behaviour – they migrated along the magnetic field lines (as seen in this Video). Unfortunately, the finding has never become known outside a narrow circle of Bellini’s colleagues, as his two papers on the phenomenon had not been published in a peer-reviewed journal1. Luckily, a rediscovery was to happen.
About 20 years later, oblivious to the Bellini’s observation, a young American microbiologist Richard P. Blakemore marvelled at the magnetically guided movement of enigmatic bacteria, which he named magnetotactic2. This marked the beginning of an adventurous journey of scientists from a broad spectrum of disciplines, microbiology, physics, biophysics, bio- and nanotechnology, as they embarked on the studies of this new group of organisms. It turned out that the magnetic navigation of magnetotactic bacteria (MTB) is enabled by biosynthesis of one or several chains of specialized magnetic organelles called magnetosomes. Each magnetosome consists of a nanosized (~37 nm) magnetic crystal, typically composed of magnetite (Fe3O4), encapsulated within a lipid bilayer membrane with incorporated specialized proteins (Fig. 1A, B). Together, they form a sophisticated navigation device, analogous to a compass needle, which MTB use to accelerate the search for favourable conditions within the stratified aquatic sediments.
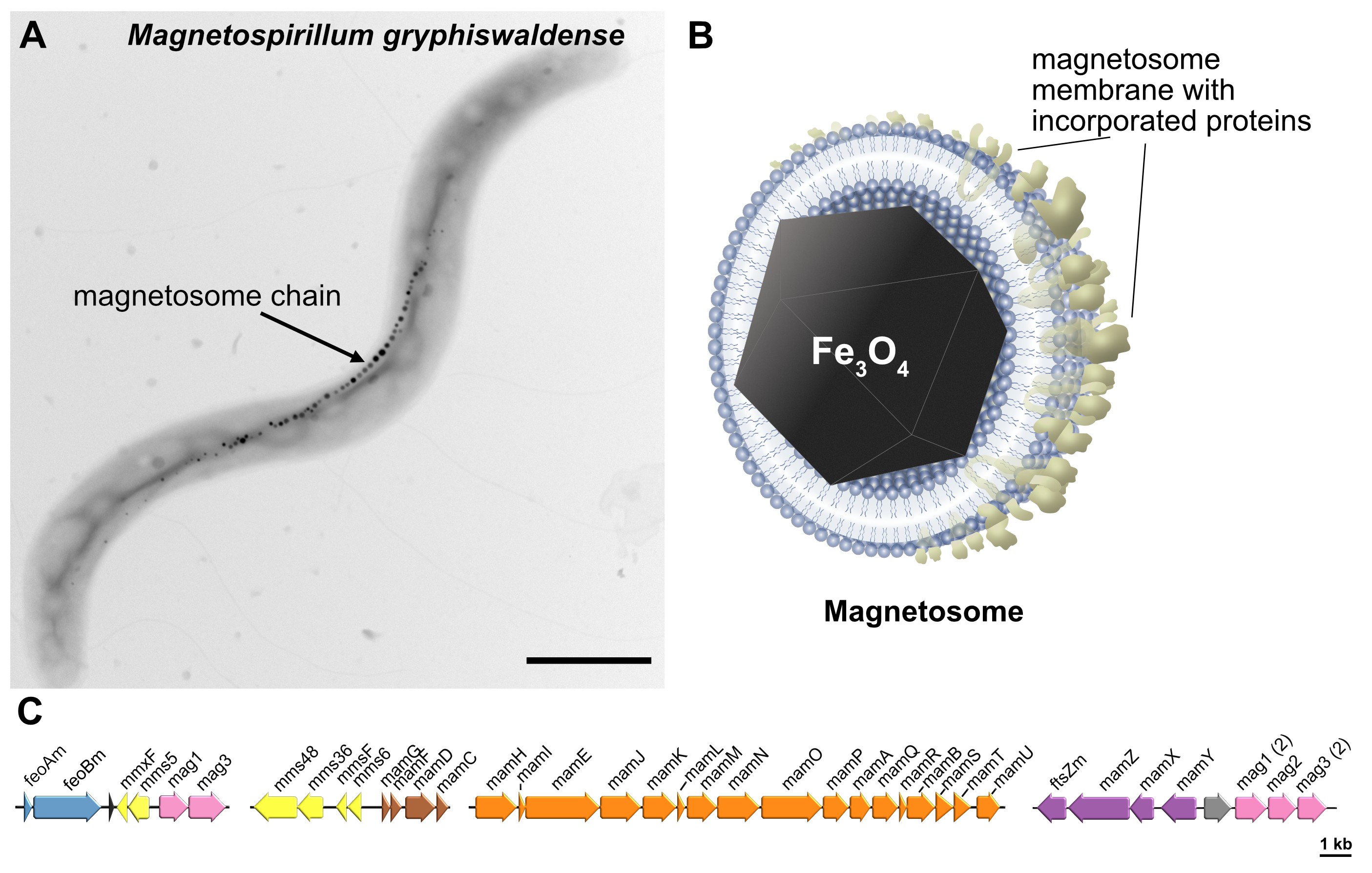
Fig. 1 Magnetosomes of magnetotactic bacteria: sophisticated magnetic organelles and promising nanomaterial. (A) Model organism Magnetospirillum gryphiswaldense. Scale bar: 1 µm. (B) Schematic of a magnetosome. (C) Magnetosome gene cluster.
Magnetosomes: Sophisticated Magnetic Organelles and Promising Nanomaterial
Further studies revealed an astonishing complexity of the magnetosome formation, defying the conventional perception of prokaryotic cells as simple entities. Altogether, more than 30 dedicated genes have turned out to be directly responsible for magnetosome formation (Fig. 1C), and at least a dozen other genes controlling general metabolic functions that additionally contribute to it.
Concurrently with the discoveries on the mechanisms behind magnetosome formation, it became increasingly evident that magnetosomes represent an exceptional nanomaterial for nanobiotechnological applications. Their attractiveness is due to high chemical purity, crystalline structure, favourable size range, colloidal stability and inherent biocompatibility, thanks their biological membrane coating3. Furthermore, their surface can be readily functionalized through genetic engineering of magnetosome proteins4.
However, despite the academic success in developing magnetosome-based diagnostic and therapeutic methods, the finicky nature of MTB has long hindered the translation of this material into applicable technologies. This is where synthetic biology enters the stage, offering new avenues for magnetosome exploration.
Harnessing Magnetosome Production through Synthetic Biology
The idea to genetically transfer magnetosome formation into more readily cultivable bacteria has been around from the initial discovery of the relevant genes. However, the genetic and mechanistic complexity of the magnetosome formation hampered the engineering of foreign strains for magnetosome production. After years of painstaking effort, a breakthrough was achieved with the creation of the first robust magnetosome-producing strain, Rhodospirillum rubrum 'magneticum,' derived from the photosynthetic alphaproteobacterium R. rubrum5. Later, a couple of other strains, while less proficient in magnetosome synthesis, were also successfully constructed6,7. Nevertheless, numerous other tested hosts failed to produce magnetosomes upon gene transfer, highlighting the ongoing reliance on the trial-and-error approach in host selection.
This prompted us to systematically explore the potential of various bacteria for magnetosome formation following gene transfer. In our study, we assessed 25 proteobacterial hosts spanning different phylogenetic groups within Alpha-, Beta-, Gamma- and Epsilonproteobacteria, by introducing the entire set of the magnetosome genes from the well-studied magnetotactic organism Magnetospirillum gryphiswaldense (Fig. 1A, Fig. 2A). Our selection criteria for the strains were stringent: the chosen hosts had to be capable of thriving in low oxygen environment, easily culturable, belong to class Proteobacteria (to minimize extensive genetic modifications), and have sequenced genomes to facilitate the mutant’s verification.
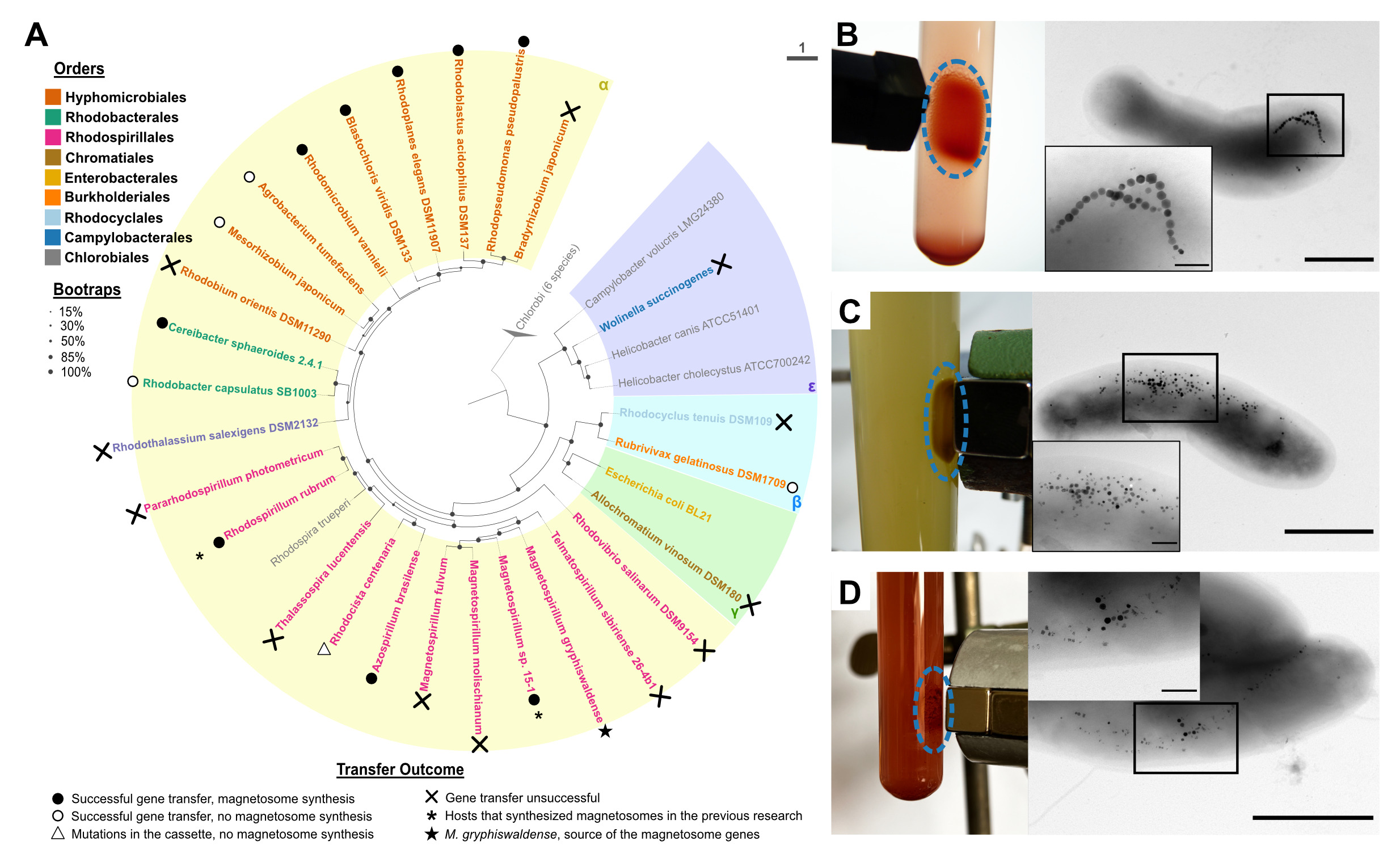
It is worth noting that only a few of these prospective hosts were domesticated and had established genetic systems. For most strains, additional development of the manipulation methods was necessary. This extensive experimentation culminated in the creation of seven new magnetosome-producing strains based on the following species: Cereibacter (Rhodobacter) sphaeroides, Rhodoplanes elegans, Rhodopseudomonas pseudopalustris, Blastochloris viridis, Rhodoblastus acidophilus, Azospirillum brasilense, and Rhodomicrobium vannielii. Among the remaining strains, five were unable to produce magnetosomes despite receiving intact genes, in another strain, the genes underwent deleterious mutations, and twelve resisted receiving the magnetosome genes. Nonetheless, our approach considerably expanded the repertoire of transgenic magnetosome producers.
Distinct Magnetosome Properties in Magnetized Strains
As the successful hosts produced magnetosomes that varied in crystal properties and intracellular arrangement (Fig. 2B-D), we collaborated with Prof. Dr. Mihály Pósfai from the University of Pannonia in Hungary to characterize their composition and properties.
Among the magnetized strains, Cereibacter (Rhodobacter) sphaeroides, Rhodoplanes elegans, and Azospirillum brasilense tended to produce relatively scarce and small magnetosome crystals. In contrast, magnetosomes from Rhodoblastus acidophilus and Rhodomicrobium vannielii approached both the abundance and sizes found in the parental organism, M. gryphiswaldense (35.93 ± 9.42 nm and 30.44 ± 9.95 nm respectively).
Two other hosts, Rhodopseudomonas pseudopalustris and Blastochloris viridis, also exhibited prolific magnetosome synthesis. However, the majority of their magnetosomes displayed an aberrant, “flake-like” crystal morphology (Fig. 2C, D). We also observed that C. sphaeroides tended to form more twinned and tripled magnetite crystals, while magnetosome nanoparticles in the natural host typically consist of a single crystal.
What Makes a Good Host for Magnetosome Biosynthesis?
We were elated to see that so many hosts were able to produce magnetosomes. However, some other seemingly similar strains, Rhodobacter capsulatus, Mesorhizobium japonicum, Agrobacterium tumefaciens, and Rubrivivax gelatinosus, could not do it despite the stable integration of the magnetosome genes into their genomes. This led us to ponder whether there were defining characteristics that successful hosts had in common. This question, however, was far from straightforward, given that not all the factors contributing to magnetosome formation are fully understood. We decided to investigate it by comparing the genomic and metabolic features of these hosts.
As we delved into the genes present in all magnetized bacteria but absent in their non-magnetized counterparts, a clear-cut answer remained elusive. There was no single gene, or group of genes universally present in successful hosts while absent in unsuccessful strains. While some individual genes missing in each non-magnetized species might be relevant, we still lack experimental data to definitively link them to magnetosome formation. Nonetheless, if we zoom out and look more broadly at the metabolic features typical to the successful hosts, we see that they all are capable of efficient anaerobic growth, sustained either by photosynthesis or denitrification. Interestingly, in the gene donor M. gryphiswaldense, magnetosome biosynthesis occurs under anaerobic conditions and with low oxygen concentrations (1-2%), whereas in the magnetized strains, it requires anaerobic conditions. It is not yet clear, what defines these differences or what photosynthesis, denitrification, and magnetosome biosynthesis have in common. Future research will hopefully untangle these relationships.
In summary, the diversity of the created magnetized strains promises to facilitate the transition of magnetosomes from academic endeavour to application. Now, we can select organisms with the preferred properties of both the magnetosomes and the host’s metabolic background, depending on the application. For instance, C. sphaeroides is known for its lack of endotoxin, making it preferred strain to produce endotoxin-free superparamagnetic nanoparticles for medical applications, whereas R. acidophilus and R. vannielii can become cost-efficient sources for isolation of magnetosomes with properties close to native magnetotactic bacteria.
For more details of our work, please see the original article "Exploring the host range for genetic transfer of magnetic organelle biosynthesis" in Nature Nanotechnology.
Acknowledgements
I would like to thank my colleagues at the Schüler Lab, especially Prof. Dr. Dirk Schüler for his mentorship and Dr. Frank Müller for his valuable contribution to the project and giving feedback to this post. Special thanks to Prof. Dr. Mihály Pósfai for the crystallography analysis. I am also grateful to our technicians Julia Kachel and Brigitte Melzer and to my students A. Hübner and L. Borgert for their help with the experiments.
References
- Frankel, R. B. The discovery of magnetotactic / magnetosensitive bacteria. Chinese J. Oceanol. Limnol. 27, 1 (2009).
- Blakemore, R. Magnetotactic bacteria. Science 190, 377–379 (1975).
- Uebe, R. & Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 14, 621–637 (2016).
- Mickoleit, F. & Schüler, D. Generation of Multifunctional Magnetic Nanoparticles with Amplified Catalytic Activities by Genetic Expression of Enzyme Arrays on Bacterial Magnetosomes. Adv. Biosyst. 2, 1700109 (2018).
- Kolinko, I. et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat. Nanotechnol. 9, 193–197 (2014).
- Dziuba, M. V., Zwiener, T., Uebe, R. & Schüler, D. Single-step transfer of biosynthetic operons endows a non-magnetotactic Magnetospirillum strain from wetland with magnetosome biosynthesis. Environ. Microbiol. 22, 1603–1618 (2020).
- Dziuba, M. V. et al. Silent gene clusters encode magnetic organelle biosynthesis in a non-magnetotactic phototrophic bacterium. ISME J. 17, 326–339 (2023).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in