Fueling the failing heart: Meta-analysis identifies metabolic treatment strategies for heart failure.
Published in Cell & Molecular Biology and General & Internal Medicine
Introduction
Despite advances in modern treatment, heart failure (HF) remains a significant health and economic burden. A key, yet often underappreciated aspect in the pathophysiology of HF is metabolic dysfunction. Under normal conditions, the heart derives most of its energy from fatty acid oxidation (FAO) and glucose oxidation (GO). In the failing heart, however, these metabolic pathways are disrupted, resulting in impaired energy production.
Emerging evidence from animal models suggests that targeting FAO and GO pathways can influence cardiac function.It has been proposed that inhibiting FAO may benefit the failing heart by promoting a shift toward the more oxygen-efficient GO. Direct stimulation of GO is also considered protective in HF. However, these findings have been inconsistent, and no systematic evidence exists to clearly guide how cardiac metabolism should be modulated to improve heart function.
What we did
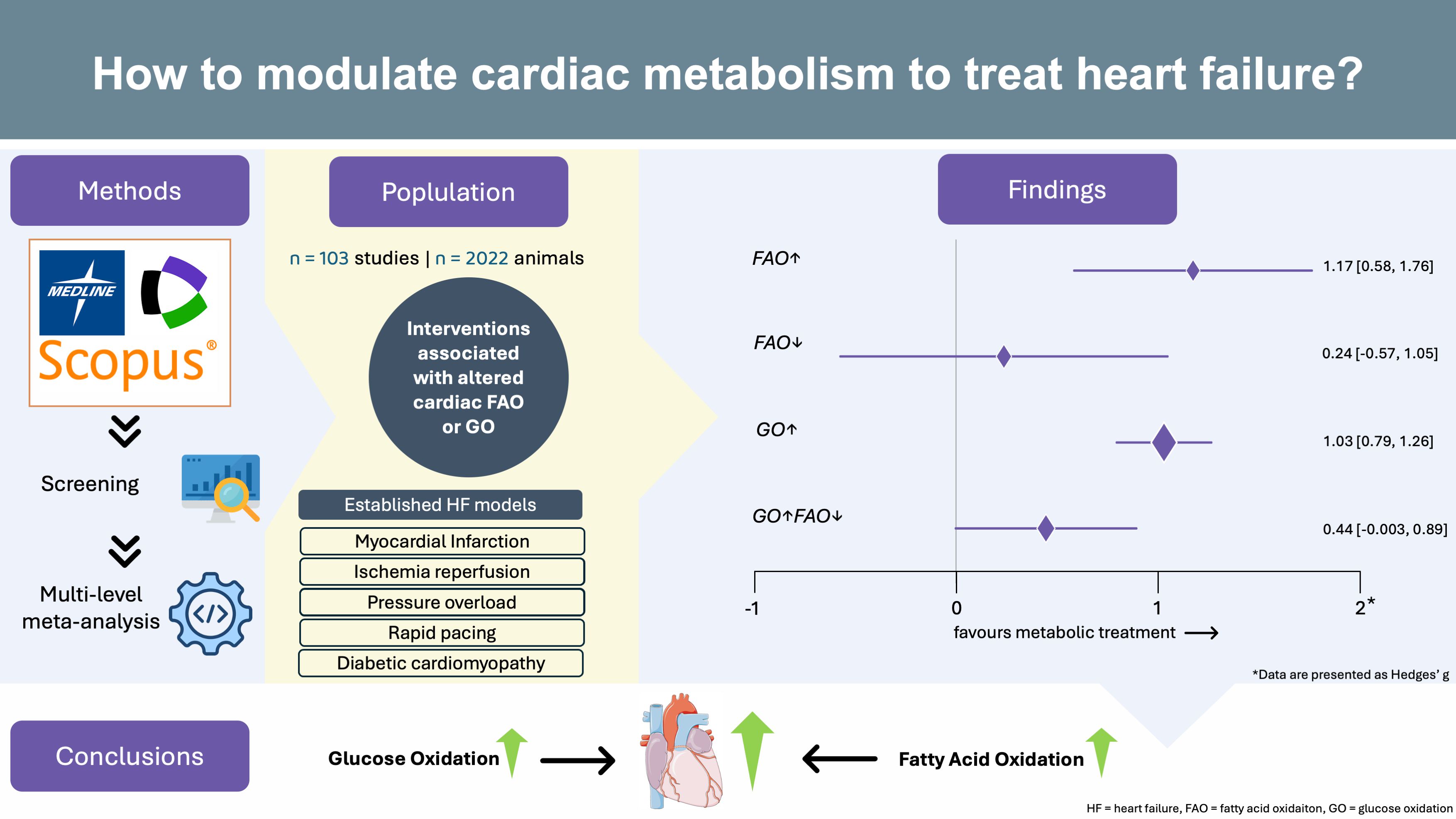
In our meta-analysis published in Communications Medicine, we evaluated how modulating cardiac GO or FAO may affect heart function in animal models of heart failure. We searched three major databases (Medline, Scopus, and Web of Science), screening more than 10,000 abstracts. After careful review, we included 103 publications that met these criteria:
- Established animal models of heart failure including: myocardial ischemia, pressure overload, or diabetic cardiomyopathy
- Direct measurements of GO or FAO: by using, for example, radiolabeled substrates. Gene or protein expression studies are not eligible. Changes in GO or FAO must be statistically significant.
- Heart function outcomes reported: at least one measure of heart function had to be reported. To ensure a comprehensive assessment of cardiac function, we included not only systolic parameters but also diastolic, morphological, and histological parameters.
Because different labs measured heart function in different ways, we used a statistical approach to translate each outcome into a standardized effect size (Hedges’ g). A multilevel random-effects model accounted for differences between studies and within-study variability.
What we found
After analyzing the data, the following findings emerged:
1. Metabolic alterations associated with improvements in cardiac funtion:
-
-
Treatments associated with enhanced cardiac fatty acid oxidation: led to remarkable improvement of cardiac function (Hedges' g = 1.17; 95% CI: 0.58-1.76; p < 0.001). Note: Hedges' g > 0.8 is considered a large effect.
- Treatments associated with enhanced cardiac glucose oxidation: showed strong and consistent benefits to cardiac function (Hedges' g = 1.03; 95% CI: 0.79-1.26; p < 0.001).
-
Treatments associated with enhanced cardiac fatty acid oxidation: led to remarkable improvement of cardiac function (Hedges' g = 1.17; 95% CI: 0.58-1.76; p < 0.001). Note: Hedges' g > 0.8 is considered a large effect.
2. Metabolic alterations not associated with improvements in cardiac funtion:
-
-
Treatments associated with the inhibition of cardiac fatty acid oxidation: led to a small and not statistically significant pooled effect size (Hedges' g = 0.24; 95% CI: -0.57-1.05; p = 0.557).
- Treatments associated with the combined inhibition of cardiac FAO and stimulation of cardiac GO: led to a moderate and nonsignificant effect (Hedges' g = 0.44; 95% CI: -0.003-0.89; p = 0.052
-
Treatments associated with the inhibition of cardiac fatty acid oxidation: led to a small and not statistically significant pooled effect size (Hedges' g = 0.24; 95% CI: -0.57-1.05; p = 0.557).
Why our findings are important
Our findings challenge the prevailling assumption that inhibiting cardiac FAO improves cardiac function in HF. We found no relevant benefit for lowering FAO across animal models. Instead, interventions that increased either FAO or GO led to remarkable improvements in heart function. These results were robust in sensitivity analyses and unlikely unaffected by publication bias
Notably, there are currently no approved drugs that may enhance cardiac GO. In case of cardiac FAO, pharmaceutical research has focused on fatty acid inhibitors so far. Therefore, our data uncover a significant gap in pharmacological development and call for the development of drugs that boost cardiac FAO or GO to improve heart function.
Take-Home Message
- Stimulating FAO or GO in the heart improves heart function in preclinical heart failure models.
- Inhibiting FAO shows no consistent benefit, questioning the current notion in metabolic therapies.
- Activators of cardiac FAO or GO should be developed to treat HF
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in