Fungi and bacteria crosstalk in the gut microbiota influences susceptibility to inflammation
Published in Microbiology

Explore the Research

Error: DOI Not Found
This DOI cannot be found in the DOI System. Possible reasons are:
The discovery of the extraordinary complexity and abundance of microbes living on the human body has triggered a significant modification of the public view of his own body. Especially with the resulting discoveries on how microbes from these microbiota influence the host health which in turn has strongly modified the perception of the public of the role of microbes in general. But the more we dig in this field the more complex the picture becomes mainly because we know so little about the diversity of the microbes and their biological properties. As a simple example, we know now clearly that not only bacterial populate the human body but archaea, bacteriophages, virus, and fungi are present in various proportions… It happens that fungi are my favorite bugs! I will thus tell you a little about a recent story we published recently in Microbiome.
So in our lab amongst other subjects, I am interested in this specific part of the microbiota: the fungal microbiota or “mycobiota”. And our main interest is to elucidate how fungal cells can influence gut inflammation encountered in the Inflammatory Bowel Diseases (IBDs) in Humans.

This work has taken place in France in the Micalis Institute at INRA (National Institute for Agronomic Research: www.micalis.fr). Micalis Institute is part of a much larger campus where many other fields of research are investigated; it is localized at Jouy en Josas in the green suburb of the West of Paris. The campus harbors different facilities including conventional and germ-free animal facilities as well as proteomics or microscopic facilities. 340 people are working at the Micalis Institute with 130 permanent researchers, engineers and technicians.
My interest in the mycobiota has begun 5 years ago while working on a specific fungal pathogen Candida albicans I decided to widen my view, focusing not only on one microorganism but on the diversity of the fungi. An encounter with Pr. Harry Sokol a gastroenterologist, also interested in this specific microbial community was the starting point of this project. Indeed, many different results from the isolation of a probiotic strain of Saccharomyces boulardii from traditional food in the 1920s to the in vitro experiments on the pro-inflammatory function of the gut colonizer Candida albicans, pointed to a potential effect of fungi in gut inflammation. With Harry Sokol and his collection of 235 samples from IBD patients, we were the first to show without a doubt that the mycobiota was clearly modified during gut inflammation (Sokol et al. Gut 2016). Additionally, this study was used to follow the potential interactions and co-occurrence of bacteria and fungi in the gut showing that depending on the disease the two populations reacted differently.
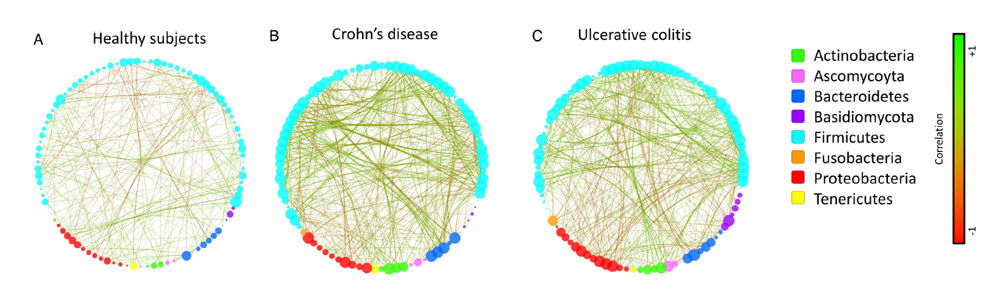
This was the foundation of our recent publication in Microbiome: we decided to focus our research on bacterial-fungal interactions in a disease context of gut inflammation.
All the experiments were done in our conventional animal facility in the campus on C57BL/6 female mice aged 8-9 weeks. The approach we choose on this project was the modification of the bacterial microbiota by antibacterial treatments, the gavage of different fungi and to trigger the intestinal inflammation using a well-described chemical method using dextran sodium sulfate (DSS) in the drinking water. We first verified that as mentioned in the literature, Candida albicans increases the susceptibility of mice to colitis induced by DSS and that Saccharomyces boulardii CNCM I-745 had the opposite effect and decrease the inflammation and the symptoms of colitis. Interestingly using broad-spectrum antibiotic we showed that in this condition the DSS treatment is not enough to trigger colitis. Tests of each antibiotic independently showed that mainly Gram-positive bacteria targeted by vancomycin were required for the inflammation induced by DSS probably through different mechanisms involving the host immune system. Additionally, in this setting with no colitis, C. albicans had no effect on the inflammation suggesting that the C. albicans needs an inflammation at least at its beginning to affect the susceptibility of the mice.

On the other hand we showed that colistin treatment suppressed the effect of fungi on the susceptibility to DSS induced colitis. Indeed, if colistin treated mice were treated with C. albicans or S. boulardii no modification of the severity of the colitis was observed contrary to what we observed when mice received no antibiotics at the beginning of our study. These data suggested that colistin modified the bacterial microbiota in a way that influences the effect of fungi on the host. Using metabarcoding for the analysis of bacterial (16S) and fungal (ITS2) microbiota composition we hypothesized that Enterobacteriaceae might be the bacterial strain involved in this interaction with the fungal cells since colistin targeted mostly this family.
After several brainstorming sessions in our office with Bruno Sovran the postdoc working on this project and Harry Sokol we decided that one way of proving the importance of Enterobacteriaceae on the effect of fungi in mice depleted of Enterobacteriaceae by the colistin treatment was to introduce back Enterobacteriaceae in mice under colistin and expect to get back the phenotype observed in mice receiving no antibiotic. In order to do so, we used a strain of Escherichia coli resistant to colistin that we gave to all groups except one.

It was a exciting surprise to observe that supplementation with E. coli (Strain MCR1 - resistant to colistin) was sufficient to observe the pro- or anti-inflammatory effects of both fungal strains. During this experiment, we also quantified the level of colonization of bacteria and fungi and we were able to show that at least part of the effect of the E. coli was to increase considerably the level of gut colonization of both fungi.
Further investigations are needed to follow up on the mechanisms involved behind this effect of Enterobacteriaceae on fungal colonization and the effects on the host. Nevertheless, these results are strongly suggesting that the study of both populations together and their potential interaction is paramount in the future.
The publication is available here: https://doi.org/10.1186/s40168-018-0538-9
Follow the Topic
-
Microbiome

This journal hopes to integrate researchers with common scientific objectives across a broad cross-section of sub-disciplines within microbial ecology. It covers studies of microbiomes colonizing humans, animals, plants or the environment, both built and natural or manipulated, as in agriculture.
Related Collections
With Collections, you can get published faster and increase your visibility.
Harnessing plant microbiomes to improve performance and mechanistic understanding
This is a Cross-Journal Collection with Microbiome, Environmental Microbiome, npj Science of Plants, and npj Biofilms and Microbiomes. Please click here to see the collection page for npj Science of Plants and npj Biofilms and Microbiomes.
Modern agriculture needs to sustainably increase crop productivity while preserving ecosystem health. As soil degradation, climate variability, and diminishing input efficiency continue to threaten agricultural outputs, there is a pressing need to enhance plant performance through ecologically-sound strategies. In this context, plant-associated microbiomes represent a powerful, yet underexploited, resource to improve plant vigor, nutrient acquisition, stress resilience, and overall productivity.
The plant microbiome—comprising bacteria, fungi, and other microorganisms inhabiting the rhizosphere, endosphere, and phyllosphere—plays a fundamental role in shaping plant physiology and development. Increasing evidence demonstrates that beneficial microbes mediate key processes such as nutrient solubilization and uptake, hormonal regulation, photosynthetic efficiency, and systemic resistance to (a)biotic stresses. However, to fully harness these capabilities, a mechanistic understanding of the molecular dialogues and functional traits underpinning plant-microbe interactions is essential.
Recent advances in multi-omics technologies, synthetic biology, and high-throughput functional screening have accelerated our ability to dissect these interactions at molecular, cellular, and system levels. Yet, significant challenges remain in translating these mechanistic insights into robust microbiome-based applications for agriculture. Core knowledge gaps include identifying microbial functions that are conserved across environments and hosts, understanding the signaling networks and metabolic exchanges between partners, and predicting microbiome assembly and stability under field conditions.
This Research Topic welcomes Original Research, Reviews, Perspectives, and Meta-analyses that delve into the functional and mechanistic basis of plant-microbiome interactions. We are particularly interested in contributions that integrate molecular microbiology, systems biology, plant physiology, and computational modeling to unravel the mechanisms by which microbial communities enhance plant performance and/or mechanisms employed by plant hosts to assemble beneficial microbiomes. Studies ranging from controlled experimental systems to applied field trials are encouraged, especially those aiming to bridge the gap between fundamental understanding and translational outcomes such as microbial consortia, engineered strains, or microbiome-informed management practices.
Ultimately, this collection aims to advance our ability to rationally design and apply microbiome-based strategies by deepening our mechanistic insight into how plants select beneficial microbiomes and in turn how microbes shape plant health and productivity.
This collection is open for submissions from all authors on the condition that the manuscript falls within both the scope of the collection and the journal it is submitted to.
All submissions in this collection undergo the relevant journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief of the relevant journal. As an open access publication, participating journals levy an article processing fee (Microbiome, Environmental Microbiome). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief of the journal where the article is being submitted.
Collection policies for Microbiome and Environmental Microbiome:
Please refer to this page. Please only submit to one journal, but note authors have the option to transfer to another participating journal following the editors’ recommendation.
Collection policies for npj Science of Plants and npj Biofilms and Microbiomes:
Please refer to npj's Collection policies page for full details.
Publishing Model: Open Access
Deadline: Jun 01, 2026
Microbiome and Reproductive Health
Microbiome is calling for submissions to our Collection on Microbiome and Reproductive Health.
Our understanding of the intricate relationship between the microbiome and reproductive health holds profound translational implications for fertility, pregnancy, and reproductive disorders. To truly advance this field, it is essential to move beyond descriptive and associative studies and focus on mechanistic research that uncovers the functional underpinnings of the host–microbiome interface. Such studies can reveal how microbial communities influence reproductive physiology, including hormonal regulation, immune responses, and overall reproductive health.
Recent advances have highlighted the role of specific bacterial populations in both male and female fertility, as well as their impact on pregnancy outcomes. For example, the vaginal microbiome has been linked to preterm birth, while emerging evidence suggests that gut microbiota may modulate reproductive hormone levels. These insights underscore the need for research that explores how and why these microbial influences occur.
Looking ahead, the potential for breakthroughs is immense. Mechanistic studies have the power to drive the development of microbiome-based therapies that address infertility, improve pregnancy outcomes, and reduce the risk of reproductive diseases. Incorporating microbiome analysis into reproductive health assessments could transform clinical practice and, by deepening our understanding of host–microbiome mechanisms, lay the groundwork for personalized medicine in gynecology and obstetrics.
We invite researchers to contribute to this Special Collection on Microbiome and Reproductive Health. Submissions should emphasize functional and mechanistic insights into the host–microbiome relationship. Topics of interest include, but are not limited to:
- Microbiome and infertility
- Vaginal microbiome and pregnancy outcomes
- Gut microbiota and reproductive hormones
- Microbial influences on menstrual health
- Live biotherapeutics and reproductive health interventions
- Microbiome alterations as drivers of reproductive disorders
- Environmental factors shaping the microbiome
- Intergenerational microbiome transmission
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jun 16, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in