Go with the Flow: How Fluid Flow Shapes Immune Responses in a Tendon-on-a-Chip
Published in Bioengineering & Biotechnology, Immunology, and Anatomy & Physiology
The challenge of studying tendon healing
Tendon injuries are a widespread clinical problem, affecting millions each year and often leading to chronic pain and reduced mobility. One of the major challenges in tendon repair is the inflammatory response triggered by the body's immune system. Blood vessels that invade the injured tendon bring immune cells that can either support healing or contribute to peritendinous adhesions and fibrosis, a scarring process resulting in excess extracellular matrix that weakens the tissue. Understanding this balance is crucial—but studying it in humans has been difficult due to a lack of physiologically relevant models.
Building a better model: The fluidic tendon-on-a-chip
To address this, we developed a fluidic human tendon-on-a-chip (hToC), a micro-engineered platform that mimics the vascular environment of an injured tendon. Tissue chips, or microphysiological systems, have emerged as promising technologies to model miniaturized functional units of human organ systems. Unlike traditional 2D cell cultures in a petri dish or animal models, tissue chips better mimic human physiology by providing conditions that replicate the 3D structure, mechanical forces, and biochemical signals found in real tissues, allowing for more accurate studies of disease, drug responses, and cell interactions.
Our previous tendon-on-a-chip model replicated key features of tendon healing, but it lacked one critical component—fluid flow. In real blood vessels, shear stress from flowing blood influences immune cell behavior, affecting their ability to adhere to the vessel wall and migrate into the tissue. By introducing controlled fluid flow into our model, we created a more realistic system to study these interactions.
Simplifying tissue chip design: modular, scalable, and easy to use
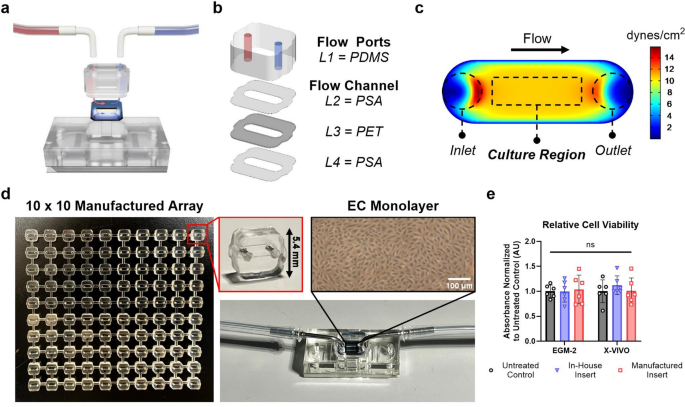
One key advantage of employing tissue chip models is the ability to create unique architectures that isolate specific cellular interactions and allow us to ask important biological questions. Microfluidic chips are often developed within the lab using specialized equipment and with methods that are difficult to reproduce. Our goal was to create components that can be built like Legos: easy to assemble and enabling customizable configurations based on the particular study. With this goal in mind, we set out to design a fluidic-integrated device with ALine, a microfluidics engineering company. Our initial design included additional features and complex geometries, so ALine engineers provided useful suggestions for adapting our design for manufacturing while maintaining essential functionality.
The ‘flow insert’ component is comprised of a flow channel to seed endothelial cells that mimic the vascular wall of blood vessels and to introduce circulating immune cells. It can be produced in quantities of hundreds to thousands of parts at a time and is easily integrated in the tendon-on-a-chip with a peel-and-stick adhesive. The peel-and-stick capability has proven extremely useful for others within and outside the lab to quickly adopt the fluidic configuration. Collaborators have begun to use the manufactured inserts in applications such as T-cell migration and neutrophil trafficking across the blood-brain barrier. Looking forward to future developments, it will now be possible to connect multiple fluidic devices to increase throughput and study crosstalk between different organ chip systems, such as between the brain and lung.
Key findings: How fluid flow shapes immune cell behavior
After establishing the flow insert, we applied physiological flow conditions to our tendon injury model. Our study revealed that shear stress plays a major role in regulating endothelial and immune cell activity within the vascular channel of the hToC:
- Endothelial cells (which line blood vessels) become activated under flow in inflammatory conditions, increasing their expression of adhesion molecules like ICAM-1, which helps recruit immune cells.
- Circulating monocytes adhere more readily and migrate into the tendon tissue under physiological shear stress, mimicking real immune responses in injured tendons.
- Tissue-resident macrophages amplify immune cell infiltration, highlighting their role in shaping inflammation and fibrosis.
These insights emphasize the importance of vascular flow in tendon healing and provide a more accurate model for studying immune-driven inflammation in soft tissue injuries.
A step toward better treatments
By integrating vascular flow into the hToC, we now have a platform that closely replicates immune dynamics in tendon injuries. This could pave the way for testing new drugs that target excessive inflammation and fibrosis, ultimately improving recovery outcomes for patients with chronic tendon disease. Beyond tendons, our model could also be adapted to study vascular inflammation in other tissues, expanding its impact across regenerative medicine.
What’s next?
In future work, we plan to use the fluidic hToC to explore potential therapies that regulate immune responses and prevent fibrosis. By studying how different treatments affect monocyte behavior under flow, we hope to uncover new strategies for improving tendon repair and reducing the burden of chronic injuries.
References
[1] Wu, F., Nerlich, M. & Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2, 332–342. https://doi.org/10.1302/2058-5241.2.160075 (2017).
[2] Tempfer, H. & Traweger, A. Tendon vasculature in health and disease. Front. Physiol. https://doi.org/10.3389/fphys.2015.00330 (2015).
[3] Low, L.A. & Tagle, D.A.. Tissue chips – innovative tools for drug development and disease modeling. Lab on a Chip 17, 3026–3036. https://doi.org/10.1039/c7lc00462a (2017).
[4] Ajalik, R. E. et al. Human tendon-on-a-chip for modeling the myofibroblast microenvironment in peritendinous fibrosis. Adv. Healthc. Mater. https://doi.org/10.1002/adhm.202403116 (2024).
Acknowledgements
Animations were adapted from Erik Patak (https://erikpatak.com/about)
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in