Growing functional kidney proximal tubules from stem cells
Published in Bioengineering & Biotechnology

Affecting more than 800 million people worldwide, chronic kidney disease (CKD) is a leading cause of death globally. Despite its prevalence, treatment options for CKD are limited and its management is complicated by our incomplete understanding of disease mechanisms. The vulnerability of the kidney proximal tubule (PT) segments to injury and the increased risk this poses for CKD development have made the generation of functional PT a key ambition of the kidney regeneration. A renewable source of mature functional human PT would prove invaluable, not only for modelling of kidney development and disease, but also as a platform for drug screening and bioengineering approaches for supplementing kidney function in affected patients.
To date, the majority of modelling and screening approaches in the kidney field have utilized 2D cultures of isolated human or rodent PT. However, they have faced similar challenges in maintaining PT maturity. Adequate recapitulation of the complex spatial organization of the PT has proven similarly difficult, with the in vivo tubule possessing distinct cell subtypes arranged throughout early, middle, and late PT segments, reflecting their differing contributions to the transitioning reabsorption, secretion, and metabolic activities of the nephron. Consequently, the advent of directed differentiation protocols that bioengineer kidney tissue (kidney organoids) from pluripotent stem cells (hPSCs) have brought great promise to the field, spurring an avalanche of studies applying kidney organoids to everything from modelling disease and toxicity to investigating cell interactions. However, the bold claims and promising findings of such studies have been tempered by the reality that kidney organoid models remain substantially immature compared to their human in vivo counterparts, particularly with respect to the PT segment.
It is this need for more accurate models of the human PT that underpinned our original report of PT-enhanced kidney organoids in 2022 1 and subsequent publication of this methodology in Nature Protocols 2. This recent manuscript provides a comprehensive insight into all aspects of PT-enhanced organoid generation, including the often overlooked early stages of PSC seeding that are critical to differentiation outcome. In developing the protocol, we considered the significant temporal differences between nephrogenesis commencement in the permanent metanephric kidney compared to current kidney organoid models, with nephron formation in vivo not commencing until ~7 weeks gestation (compared to ~1-2 weeks of differentiation in most organoid protocols). We also considered the precisely timed shifts in growth factor signaling that guide metanephric nephron progenitor specification in vivo. While subtle changes in signaling and gradient establishment are understandably difficult to recreate in an in vitro setting, this likely affects both progenitor identity and the patterning of the resulting nephrons.
Through this developmentally-informed approach, we improved PT specification and maturation within our organoid model by initially driving the posteriorisation of hPSC-derived primitive streak and intermediate mesoderm (IM) to more robustly pattern towards posterior IM-derived metanephric nephron progenitors. We then extended this metanephric specification phase, encouraging the enrichment of committed nephron progenitors through prolonged culture and prevention of precocious nephrogenesis in specialized culture media (nephron progenitor maintenance media, CDBLY2, adapted from Tanigawa et al. 3) (Figure 1). Combined, these calculated adjustments gave rise to organoids with the most mature PT reported to date when compared to all other publicly available kidney differentiation datasets, displaying the first evidence of S1, S2 and S3 PT cell subtypes, along with improved transporter functionality, and suitability for modelling PT-relevant diseases 1.
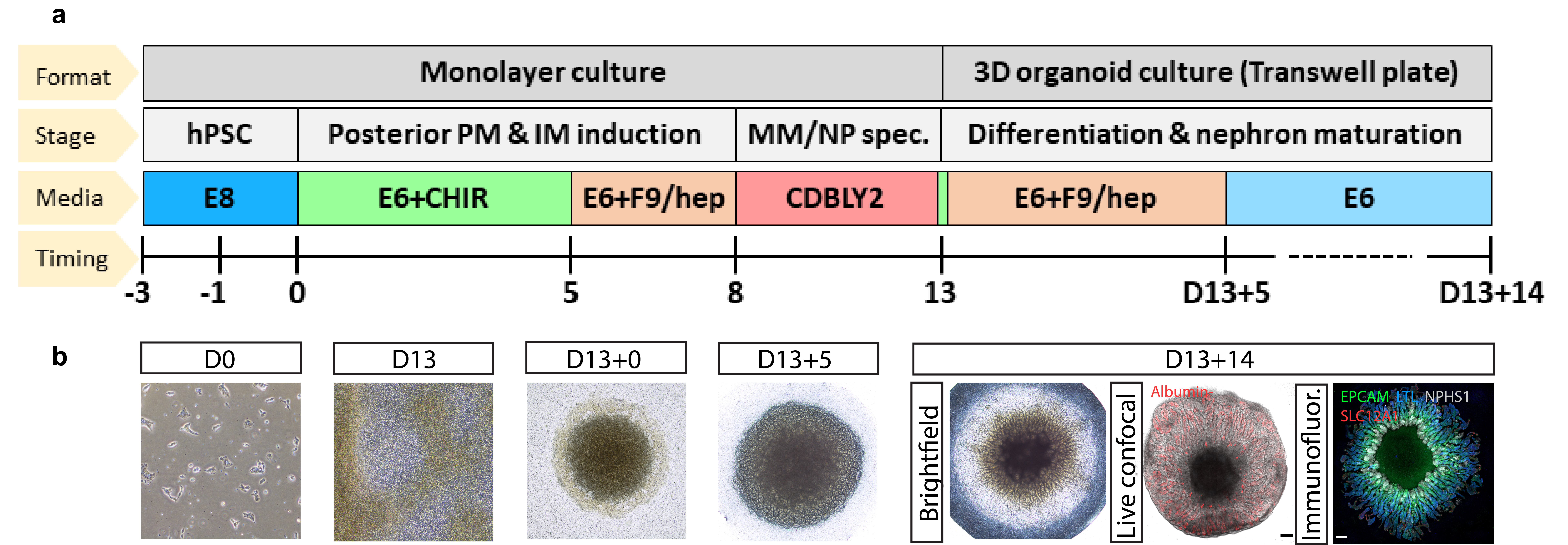
Figure 1: PT-enhanced organoid protocol and outcome. a) Schematic depicting the PT-enhanced protocol, involving initial posteriorisation of the forming primitive streak and intermediate medosderm populations through exposure of hPSCs to CHIR99021 for 5 days before the FGFG9 switch. The nephron progenitor specification phase is then initiated through prolonged culture in CDBLY2 which simultaneously prevents spontaneous epithelialisation of the progenitors whilst supporting ongoing proliferation and metanephric specification. b) Brightfield and confocal imaging (live confocal and immunofluorescence [far right]) of monolayer and organoid differentiation morphologies during key stages of the PT-enhanced protocol. Images of differentiating monolayer iPSCs and 3D organoids are depicted at days 0 (D0), 13 (D13 and D13+0), 18 (D13+5), and 27 (D13+14) of the protocol. Examples of PT-enhanced organoids of the day of harvest (D13+14) illustrate the uptake of TRITC-conjugated albumin (red) into proximal tubules, indicative of functionality, and the expression of nephron markers (nephron epithelium, EPCAM [green], proximal tubule, LTL [blue], podocytes of the glomeruli, NPHS1 [grey], and loop of Henle, SLC12A1 [red]). Scale bars represent 200µm.
Intriguingly, within 5 – 8 days of creating a micromass from these metanephric progenitors, the resulting PT-enhanced organoids developed striking radially aligned nephrons extending outwards from a central ring of glomeruli that surrounded an unpatterned stromal core. Detailed single-cell transcriptional profiling analyses and functional assays to establish potential causative signaling gradients, we were able to determine that nephron spatial arrangement in PT-enhanced organoids was arising from a localized source of WNT antagonism originating from a central core of cortical stroma and pre-cartilage cells. To our knowledge, this represents the first demonstration of spatially controlled nephrogenesis in bioengineered tissues.
Our detailed methodology in Nature Protocols provides deeper insights into novel approaches for improving and controlling the patterning of bioengineered kidney tissue, providing a platform on which to extend current organoid protocols and applications. It is our hope that the continued accessibility of such protocols will drive further advances in the kidney disease research field to improve CKD treatments and outcomes.
References
- Vanslambrouck JM, Wilson SB, Tan KS, Groenewegen E, Rudraraju R, Neil J, Lawlor KT, Mah S, Scurr M, Howden SE, Subbarao K, Little MH. Enhanced metanephric specification to functional proximal tubule enables toxicity screening and infectious disease modelling in kidney organoids. Nat Commun. 2022. 8;13(1):5943.
- Vanslambrouck JM, Tan KS, Mah S, Little MH. Generation of proximal tubule-enhanced kidney organoids from human pluripotent stem cells. Nat Protoc. 2023. (Epub ahead of print).
- Tanigawa S, Taguchi A, Sharma N, Perantoni AO, Nishinakamura R. Selective In Vitro Propagation of Nephron Progenitors Derived from Embryos and Pluripotent Stem Cells. Cell Rep. 2016. 26;15(4):801-813.
Contributors
Vanslambrouck JM1,2, Little MH1,2,3
- The Novo Nordisk Foundation Centre for Stem Cell Medicine (reNEW), Murdoch Children's Research Institute, Parkville, Melbourne, Australia.
- Department of Paediatrics, Faculty of Medicine, Dentistry and Health Sciences, The University of Melbourne, Parkville, Melbourne, Australia
- Novo Nordisk Foundation Centre for Stem Cell Medicine (reNEW), Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in