Hearing from our Cretaceous ancestors: a remarkable fossil discovery in Jehol Biota
Published in Ecology & Evolution

Ever wondered how mammals, including humans, developed their extraordinary hearing abilities? Join us on a journey that delves into the evolution of our middle ear, tracing it back from its ancient "reptilian" ancestors to modern mammals. At the heart of this story lies a pivotal question: where did the tiny but crucial bones of the middle ear, the malleus, incus, and stapes, originate? Contrasting with reptiles, which possess a single ear bone known as the columella, we explore the remarkable transformation of the mammalian middle ear, a subject that has been a textbook example of evolution and fascinated evolutionary biologists since the 19th century. Early embryologists made pioneering contributions by examining the intricate embryonic structures of vertebrates, establishing the homology of middle ear bones between mammals and reptiles (e.g., Meckel, 1820; Reichert, 1837; Gaupp, 1898). This foundational concept, known as the Reichert-Gaupp theory (see reviews by Anthwal et al, 2013; Maier and Ruf, 2016), was later reinforced by fossil discoveries of non-mammalian synapsids (e.g., Broom, 1904; Goodrich, 1930). The evolution of the mammalian middle ear also was long cited as evidence supporting Haeckel's recapitulation theory, emphasizing the link between ontogeny and phylogeny.
Evolutionary Stages of Mammalian Middle Ear
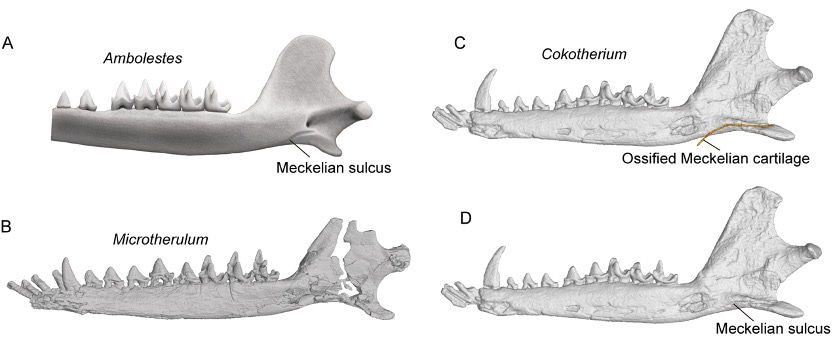
Our journey through the evolution of the mammalian middle ear leads us to explore the what, when, and how of this transformation. In contrast to the wide range of middle ear variations in modern mammals (Doran, 1876), the direct fossil evidence of the middle ear was scarce until the remarkable discoveries of Mesozoic mammals in the Early Cretaceous Jehol Biota and Middle-Late Jurassic Yanliao Biota. Within mammaliaforms, which include crown mammals and stem clades, there are three pivotal evolutionary and phylogenetic stages in the development of the mammalian middle ear: Mandibular middle ear (MME) (or Postdentary-attached ear), Meckelian-attached middle ear (MaME), and Detached middle ear (DME) (Allin, 1975; Luo, 2011; Meng, 2014; Luo and Manley, 2020; Wang et al., 2021). The groundwork for understanding the MME was laid in the 1970s and 1980s through detailed skull morphology studies, highlighting the postdentary bones and Meckel's cartilage attached to the mandible via the postdentary trough and Meckelian sulcus (Kermack, 1973, 1981). In the past two decades, the discovery of MaME in basal clades within crown mammals, such as eutriconodontans and symmetrodontans, marked significant progress in the field of paleontology and evolutionary biology (Luo, 2011; Meng, et al., 2011; Luo and Manley, 2020). The MaME is characterized by the absence of the postdentary trough and the shrinking postdentary bones conneted to the mandible through the Meckel’s cartilage. The DME emerges as the Meckelian cartilage and sulcus diminish, leading to the detachment of middle ear from the mandible.

Microtherulum: A Thrilling Discovery in Early Cretaceous
The nature of the middle ear in early therians, a clade that includes humans, remains a mystery. Our journey took a thrilling turn with the first glimpse of the specimens in the drawer in Yuanqing’s office. Our discovery was triggered by a nearly complete fossil skeleton of a small mammal preserved in counterpart slabs. This fossil, likely representing a therian mammal, was a rare find in the Early Cretaceous terrestrial Lagerstätte Jehol Biota. The excitement of the discovery was followed by meticulous preparation and high-resolution micro-CT scan. After an in-depth comparative morphological analysis, we named it as a new genus and species, Microtherulum oneirodes. The specific name, "oneirodes", pays tribute to the dreamlike nature of this fossil's discovery, which fills a critical gap in our understanding of the middle ear evolution in mammals. Our comprehensive phylogenetic analyses revealed that Microtherulum stands as one of the earliest branching eutherians at the dawn of eutherian evolution.

Saddle-shaped incudomallear joint. During virtual segmentation of CT scan data, we stumbled upon several pieces of isolated, irregular bony elements near the basicranial region. Identification and interpretation for these bones involved multiple rounds of idea exchanging among the authors, reviewers, and the editor, facilitated by data sharing. The fundamental observation in Microtherulum was the detachment of the middle ear from the mandible, with no Meckelian sulcus present on the medial side of the mandible, representing the most derived evolutionary stage of the mammalian middle ear. The most exciting discovery was the saddle-shaped articulation of two incomplete elements, identical to the malleus-incus articulation in modern therians. This discovery marks the earliest known record of the saddle-like incudomallear joint in any known Mesozoic mammaliaforms. It also confirms the presence of the typical saddle-shaped incudomallear joint in Early Cretaceous eutherians, representing a significant innovation in the mammalian middle ear. We offer a partial glimpse into the appearance of the middle ear in early therians together with the identification of the C-shaped ectotympanic and the stapes with an oval-shaped footplate in the specimens.
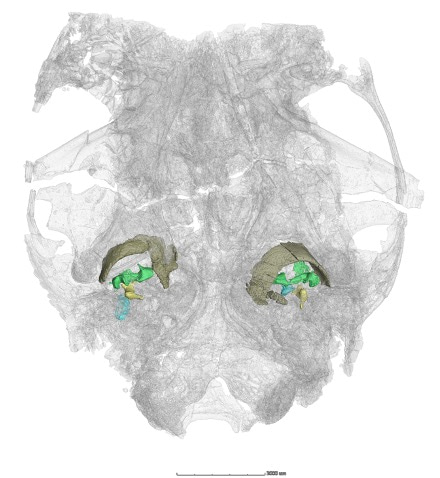
Figure 3. Virtually reconstruction middle ear in Microtherulum, in ventral view of the specimen.
Ectotympanic in brown, malleus in green, incus in yellow, and stapes in blue.
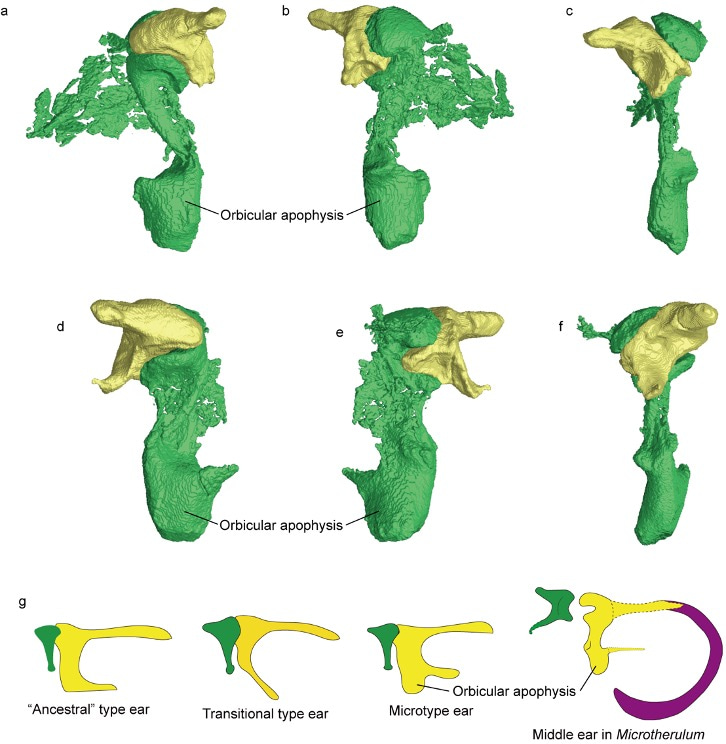
Microtype middle ear. Another fascinating discovery was the identification of the orbicular apophysis of the malleus in Microtherulum. This distinctive bone mass near the base of the manubrium is characteristic of the microtype ear in Fleischer's category (Fleischer, 1978). While distinguishing between the microtype and ancestral type ears can be challenging (Mason, 2013), the development of the orbicular apophysis in Microtherulum is more prominent than that in the ancestral type ear of opossums and the microtype ear of hedgehogs. This supports the notion that the new described middle ear is most similar to the microtype ear. Notably, this marks the first identification of the "microtype ear" in Mesozoic mammaliaforms. The discovery aligns with observations that microtype species are small mammals (e.g., bats, shrews and mice), not vice versa. The detachment of the ear, along with its functional characteristics (microtype), suggests that Microtherulum probably developed an enhanced capacity for high-frequency hearing.
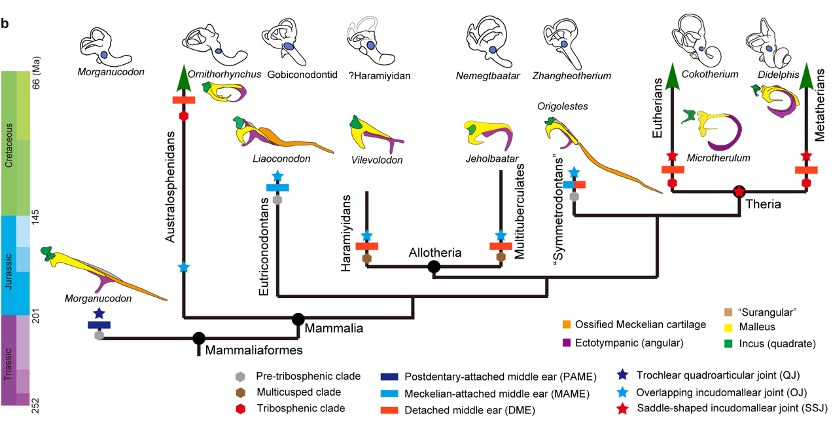
Interpreting fossil ears in Mesozoic mammaliaforms
As one of our reviewers stated: “The mammalian middle ear is one of the most difficult to interpret parts of the mammalian skull simply because a huge morphological variety exists”. Fossil ears that are over 100 million years old pose additional hurdles. State-of-the-art high-resolution imaging techniques, like synchrotron and micro/nano CT scans, have significantly improved our comprehension by overcoming obstacles in preparing and directly observing these small fossils. The incomplete nature of these delicate structures fuels ongoing debates on interpretations of fossil ears in Mesozoic mammaliaform. In the case of Microtherulum, we are fairly well-placed because both sides of the middle ear and the complete incudomallear joint are preserved, thanks to high-resolution micro CT scans (5.507 μm per pixel).
Future Insights
The detached middle ear in Microtherulum adds a layer of complexity to our understanding of middle ear variation in early therians. Notably, another Jehol eutherian, Cokotherium, discovered from the same geological formation, retained an ossified Meckelian cartilage (Wang et al., 2022). Our discovery of the microtype ear in early eutherians challenges Fleischer's hypothesis that the ancestral type ear preceded the true mammalian ear and evolved into other functional types. The ancestral states of therian ears remain an open question, raising further inquiries about how the evolutionary transformation occurred among different types of middle ears in early therians. These questions underscore the need for additional specimens to provide a comprehensive picture of the middle ear and morphological variation across various clades.
References:
Allin EF. 1975. Evolution of the mammalian middle ear. Journal of Morphology 147:403-437.
Anthwal N, Joshi L, Tucker AS. 2013. Evolution of the mammalian middle ear and jaw: adaptations and novel structures.Journal of Anatomy 222:147-160.
Broom R. 1904. On the Structure of the Theriodont Mandible, and on its Mode of Articulation with the Skull. Proceedings of the Zoological Society of London 74:490-498.
Doran AHG. 1876. Morphology of the mammalian ossicula auditus. Transactions of the Linnean Society of London1:371-497.
Fleischer G. 1978. Evolutionary principles of the mammalian middle ear. Advances in Anatomy Embryology and Cell Biology 55:1-70.
Gaupp E (1898) Ontogenese und Phylogenese des schall-leitenden Apparates bei den. Wirbeltieren. Erg Anat Entwicklungsgesch 8, 900–1149.
Goodrich ES. 1930. Studies on structure and development of vertebrates.
Kermack KA, Mussett F, Rigney HW. 1973. The lower jaw of Morganucodon. Zoological Journal of Linnean Society 53: 87-175.
Kermack KA, Mussett F, Rigney HW. 1981. The skull of Morganucodon. Zoological Journal of Linnean Society 71:1-158.
Luo ZX, Manley GA. 2020. Origins and early evolution of mammalian ears and hearing function. In: Fritzsch B, editor. The senses: a comprehensive reference. Cambridge, Massachusetts: Elsevier Academic Press. p 207-252.
Luo ZX. 2011. Developmental Patterns in Mesozoic Evolution of Mammal Ears. Annual Review of Ecology, Evolution, and Systematics 42:355-380.
Maier W, Ruf I. 2016. Evolution of the mammalian middle ear: a historical review. Journal of Anatomy 228:270-283.
Mason MJ. 2013. Of mice, moles and guinea pigs: Functional morphology of the middle ear in living mammals. Hearing Research 301:4-18.
Meckel JF (1820) Handbuch der menschlichen Anatomie, Vol. 4. Halle: Buchhandlung des Halleschen Waisenhauses.
Meng J. 2014. Mesozoic mammals of China: implications for phylogeny and early evolution of mammals. National Science Review 1:521-542.
Meng J, Wang Y, Li C. 2011. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature472:181-185.
Reichert C (1837) Ueber die Visceralbogen der Wirbelthiere im allgemeinen und. deren Metamorphosen bei den Vögeln und Säugethieren. Müllers Arch Physiol 1837, 120–222.
Wang HB, Hoffmann S, Wang DC, Wang YQ. 2022. A new mammal from the Lower Cretaceous Jehol Biota and implications for eutherian evolution. Philosophical transactions of the Royal Society of London Series B, Biological sciences 377:20210042.
Wang J, Wible JR, Guo B, Shelley SL, Hu H, Bi S. 2021. A monotreme-like auditory apparatus in a Middle Jurassic haramiyidan. Nature 590:279-283.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in