Hope and Healing: The promise of Childhood Cancer Precision Medicine Trials
Published in Genetics & Genomics

Childhood cancer is a devastating reality for thousands of families worldwide. While advances in medical science have made significant strides in treating these conditions, the quest for better, more personalized treatments continues. In recent years, precision medicine has emerged as a promising approach in pediatric oncology, offering tailored treatments based on a patient's unique genetic makeup and the specific characteristics of their cancer. This approach holds great promise for improving outcomes in childhood cancer. However, it's essential to recognize that not every patient will benefit equally from this new approach. Our recent study, titled "Hopes, Concerns, Satisfaction, and Regret in a Precision Medicine Trial for Childhood Cancer: A Mixed-Methods Study of Parent and Patient Perspectives," delves into the experiences of families participating in precision medicine trials for childhood cancer, seeking to understand the complex impacts such trials can have on families.
This study recruited participants from the ‘PRecISion Medicine for Children with Cancer’ (PRISM) trial, an Australian precision medicine trial for children with high-risk cancers as part of the Zero Childhood Cancer Program. PRISM included a comprehensive analysis of the enrolled children’s tumour cells, genetic/genomic testing of tumour and non-tumour cells, and biological models such as drug testing of tumour cells in patient-derived xenografts. The data derived from these tests was then reviewed and where possible, used to provide treatment recommendations. The recommendations were then shared with the child’s oncologist, who could choose to refine the child’s treatment in consultation with the family. The ‘PRISM-Impact’ study ran alongside PRISM and aimed to understand parents’/patients’ experiences of precision medicine. This paper reports on findings of the PRISM-Impact study.
Parents and adolescent patients completed questionnaires for the PRISM-Impact study at trial enrolment (T0) and after receiving results (T1). Parents opted-in to an interview at T1. Bereaved parents completed a questionnaire 6-months post-bereavement (T1B).
Findings
A total of 182 parents and 23 patients completed T0; 108/182 parents and 8/23 patients completed T1; 27/98 bereaved parents completed T1B; and 45/108 parents were interviewed. The study found that parents and adolescent patients harbor multifaceted hopes when enrolling in a childhood cancer precision medicine trial. These hopes include the desire to find cures not only for their child but also for future patients. Many hope that precision medicine will provide more treatment options and increase their child's chances of a cure. This mixture of altruism and personal aspiration is at the heart of the participants' motivations.
However, along with these hopes, concerns also emerge. The most common concern is the time it takes to receive results, with fears that cancer may progress during this waiting period. Other concerns include the possibility of receiving disappointing news about the aggressiveness of the cancer and the potential stress the trial might bring to their families. The figure below shows the percentage of participants that endorsed the 6 hopes (a) and 5 concerns (b) presented to them.
Figure 1. Parents’ hopes and concerns at enrolment in the PRISM trial.
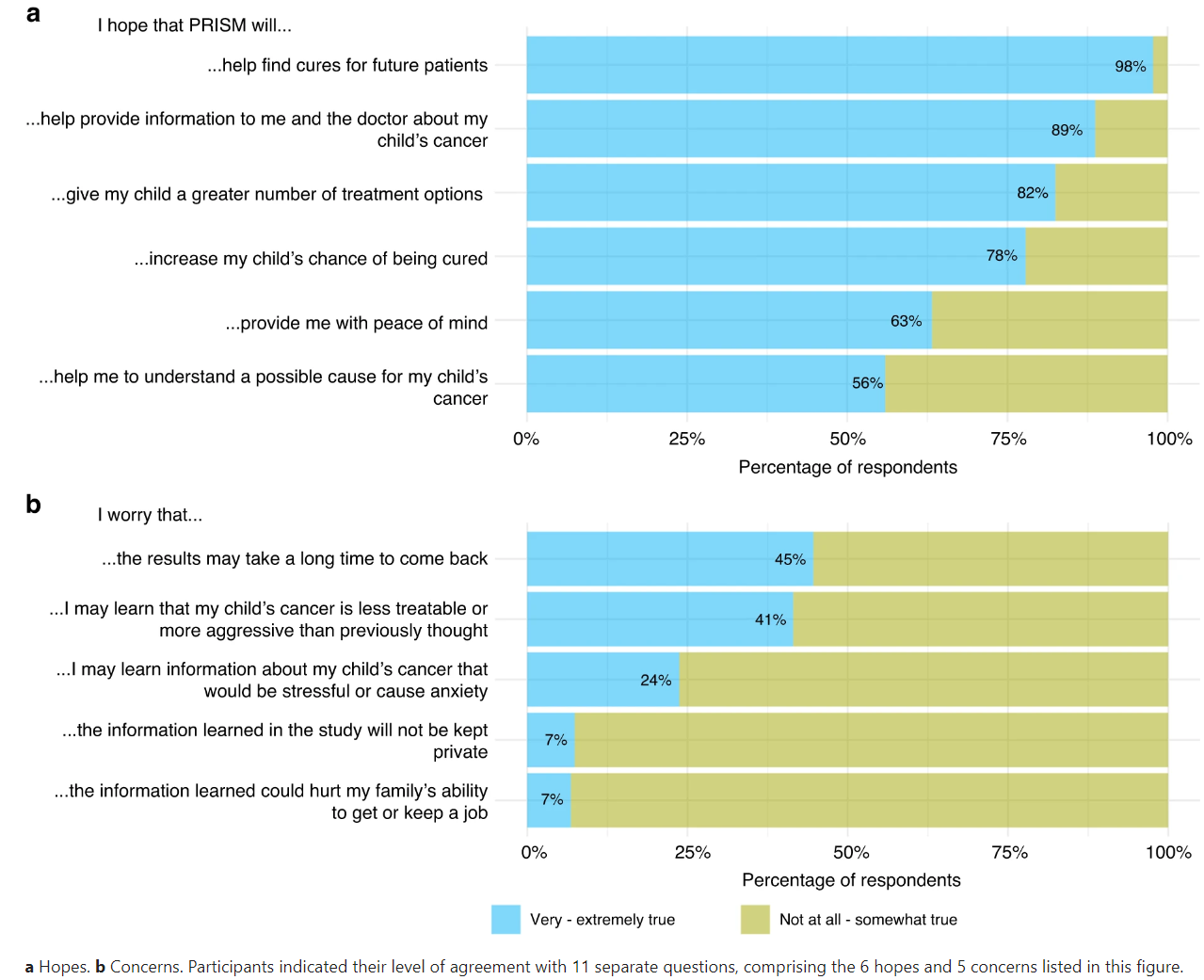
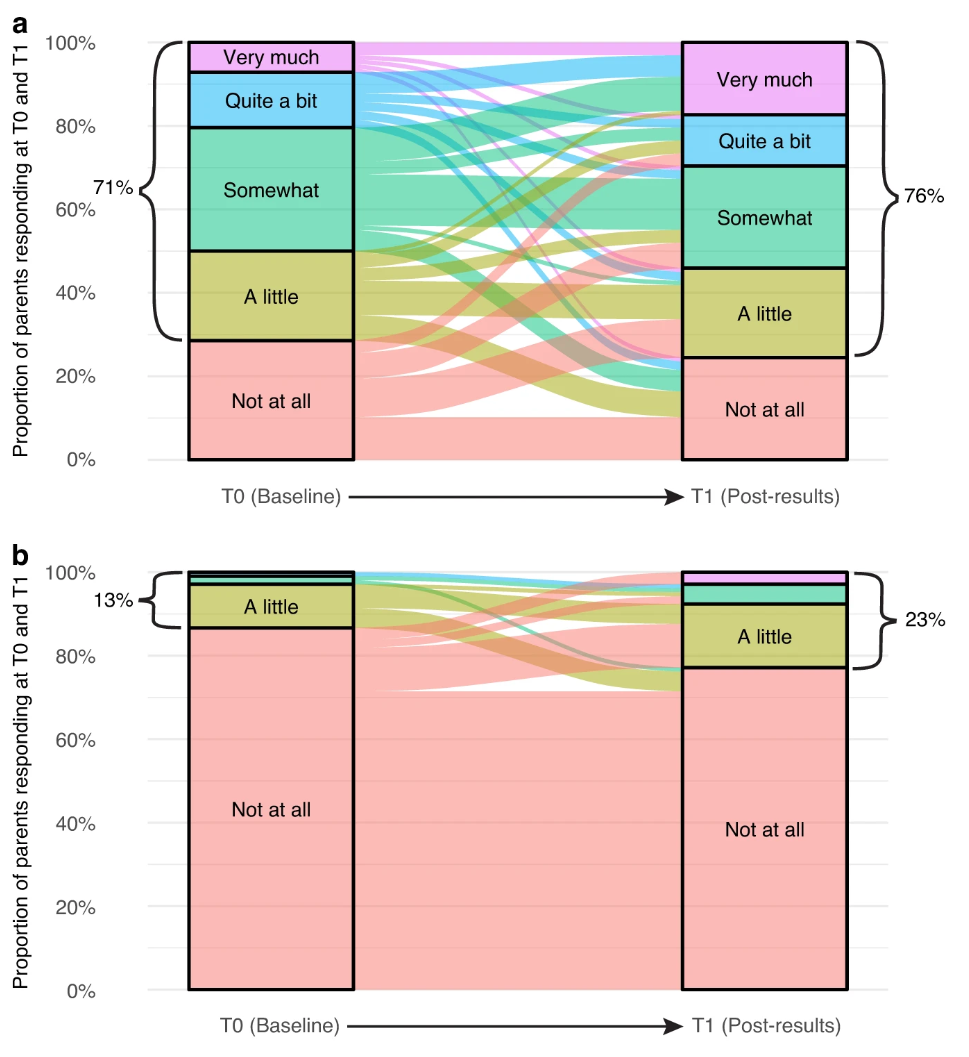
Despite these concerns, the study found that participants generally perceived benefits from their participation in the precision medicine trial. Most parents and patients reported that the benefits outweighed any burdens. For parents, the benefits included peace of mind, the feeling of having tried everything, and the hope of contributing to future cancer treatments. Even bereaved parents reported benefits, such as extra time with their child and improved quality of life. Patients, on the other hand, found comfort in knowing they were not alone in their cancer journey. The figure below shows participants perceptions of benefits (a) and burdens (b). The alluvial plots in this figure visually represent the proportion of participants’ responses to the benefit and burden questions at Time 0 and Time 1, as well as any changes in participants’ individual responses over time.
Figure 2. Parents’ perceptions of (a) benefit and (b) burden from participating in PRISM (only including those who responded at T0 and T1).
The study also explored participants' satisfaction with their decision to participate in the trial. Overall, parents expressed high levels of satisfaction at both the beginning of the trial and after the return of results. Despite some frustrations with limited communication, they remained willing to recommend the trial to others. Importantly, few parents or patients expressed regrets about their participation, even if their child did not survive. This suggests that the benefits of hope, additional time, and contributions to medical progress outweighed any potential drawbacks.
In the challenging landscape of childhood cancer, precision medicine offers a ray of hope. This study sheds light on the hopes, concerns, satisfaction, and regret experienced by parents and patients participating in precision medicine trials. Even when trial outcomes did not match their hopes, parents and patients rarely regretted participating in a childhood cancer precision medicine trial. These findings are critical for integrating participants’ views into future precision medicine delivery and underscore the importance of open communication, timely information, and continued scientific innovation to ensure that families receive the support they need during this difficult process.
This work was supported by Luminesce Alliance - Innovation for Children’s Health, which is a not-for-profit cooperative joint venture between the Sydney Children’s Hospitals Network, the Children’s Medical Research Institute, and the Children’s Cancer Institute.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in