Host–Guest Inversion Engineering Induced Superionic Composite Solid Electrolytes for High‑Rate Solid‑State Alkali Metal Batteries
Published in Bioengineering & Biotechnology, Chemistry, and Materials
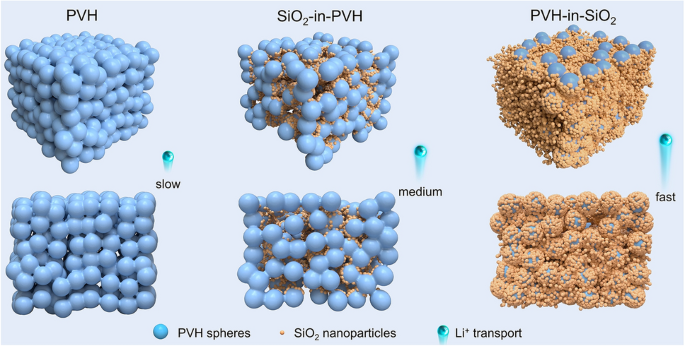
Composite solid electrolytes (CSEs) promise safer, energy-dense batteries, yet room-temperature conductivities ≥1 mS cm-1 still rely on costly, moisture-sensitive active ceramics. Now, researchers at Southeast University (China), led by Prof. Long Pan and Prof. Zheng-Ming Sun, overturn the conventional “ceramic-guest-in-polymer-host” paradigm through host–guest inversion engineering, creating a “PVH-in-SiO2” architecture that delivers 1.32 × 10-3 S cm-1 at 25 °C with only 2.9 wt % residual solvent—a 10× leap over passive-ceramic CSEs and competitive with best-in-class oxides/sulfides.
Why This Electrolyte Matters
- Superionic Highways: 158 nm SiO2 nanoparticles densely wrap interconnected PVDF-HFP micro-spheres, maximizing continuous SiO2/PVH interfaces where DFT shows Li+ diffusion barriers as low as 0.26 eV (vs 0.51 eV for SiO2/SiO2).
- Solvent-Lite: 2.9 wt % residual solvent (8–15 wt % typical) suppresses Li-metal side reactions while 87 % free-TFSI- (Raman) ensures high mobile-ion concentration.
- Universal Alkali-Ion: Identical protocol yields 3.0 × 10-4 S cm-1 (Na+) and 2.6 × 10-4 S cm-1 (K+)—14× & 64× boosts versus polymer-only analogues.
- Scalable & Flexible: 11 cm-diameter, foldable films cast from water/acetone in minutes; provisional patent filed (CN202310061247.3).
Electrochemical Performance
-
LiFePO4|PVH-in-SiO2|Li (25 °C)
– 157 mAh g-1@ 0.1C; 92.9 % retention after 300 cycles at 3C (capacity fade <0.025 % per cycle).
– High-loading (9.2 mg cm-2) cathode: 136 mAh g-1, 92 % retention/100 cycles. -
NCM622|PVH-in-SiO2|Li (4.3 V cut-off)
– 147 mAh g-1@ 0.2C, >100 cycles with 99.9 % CE. -
Na & K Full-Cells
– NVP|PVH-in-SiO2-Na|Na: 95 mAh g-1, 86 % retention/500 cycles @ 0.5C.
– KPB|PVH-in-SiO2-K|K: 84 % retention/500 cycles; PVH-K cells fail within 5 cycles.
Mechanistic Insights
SS-NMR tracking of 6Li shows 53 % of Li+ resides at SiO2/PVH interfaces after cycling (vs 2 % initially). MD simulations give Li+ diffusion coefficient 3.3 × 10-9 cm2 s-1 in PVH-in-SiO2, 3× steeper MSD slope than SiO2-in-PVH. Finite-element modeling visualizes current density 102–103 mA cm-2 localized along interfacial highways, confirming they dominate bulk transport.
Challenges & Next Steps
Thickness reduction to ≤25 µm and ceramic volume fraction optimization (currently 41 %) are underway to push areal capacity >3 mAh cm-2 with Ni-rich cathodes. Roll-to-roll coating trials with industry partners target pilot-scale pouch cells (2 Ah) by late-2025.
This host–guest inversion strategy turns low-cost, passive SiO2 into a superionic scaffold, offering a universal, solvent-lean platform for solid-state Li, Na and K batteries without the price or moisture penalties of active ceramics.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in