How calcium-regulated reprogramming primes legume root cells to welcome beneficial rhizobia symbionts
Published in Plant Science
Legumes establish unique associations with microorganisms living inside plant cells, called endosymbionts, which can provide essential nutrients for plant growth in exchange for photosynthates. As biological nutrient suppliers, these symbioses are of great interest for sustainable agriculture. The success of these interactions depends on creating privileged habitats to accommodate endosymbionts intracellularly for efficient nutrient (e.g. phosphorus or nitrogen) exchange. This can be illustrated by arbuscular mycorrhiza (AM) fungi endosymbioses, which form arbuscular hyphal structures within the root cortex, or nitrogen-fixing rhizobial root nodule endosymbioses, which incorporate rhizobia into a new nodule organ. Guiding microbial partners from the root surface to these niches is key to establishing these symbiotic exchanges. In this long journey, the plant host first selects the compatible microbial partner, mostly by recognizing chitin-based signals, before setting up a calcium-regulated pathway to orchestrate symbiotic programmes accompanying microbial root entry and further niche accommodation.
Endosymbiotic infection by rhizobia occurs in most legumes by forming the infection thread (IT)1, a new tubular structure filled with rhizobia, which grows from initial bacterial entry sites in root hairs towards the developing nodule. High-resolution microscopy in the model legume Medicago truncatula revealed that plant cells must commit to this process by acquiring a specific cell division state without cytokinesis1. By regulating IT interface construction and tracing the cellular trajectory for IT passage, plant cells also direct polarized IT progression1. This involves the formation of a cytoplasmic bridge (Fig. 1) in front of the elongating IT and in the adjacent cortical cell, which is primed to receive the growing IT. Similar cytoplasmic bridges are formed prior to AM fungal invasion2 but not during pathogenic interactions, supporting their specialization to beneficial symbionts. Although described many years ago in fixed plant tissues, little progress has been made in understanding bridge reprogramming. More recently, confocal microscopy has allowed the visualisation of cytoplasmic bridge formation in vivo in rhizobia-infected root hairs1. However, studying them in the root cortex remained challenging because of its cell specificity and transient nature, particularly difficult to access in thick, multi-layered legume root systems.
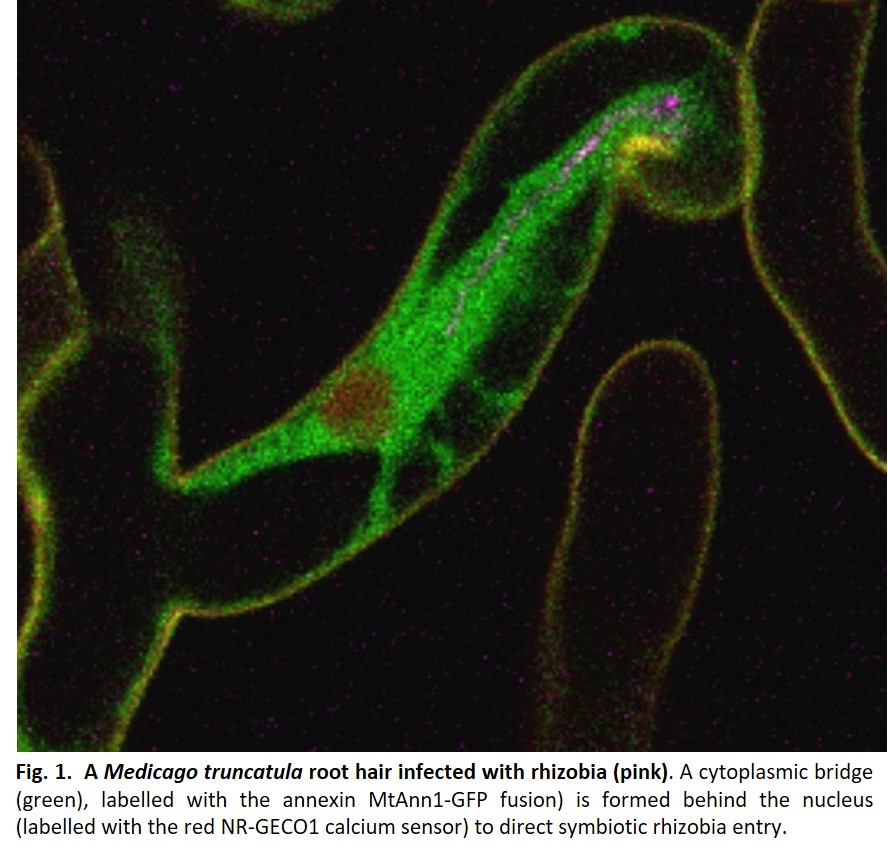
In Guillory et al. 20243, high resolution microscopy methods were combined to address cytoplasmic bridge formation during rhizobial infection in M. truncatula. This work took advantage of a previously described spot inoculation system3 to produce thin sections of rhizobia colonised root sectors. Although time consuming (~200 sections per site were made to detect one cytoplasmic priming event), basic fuchsin staining of root tissues helped visualise these events for further ultrathin sectioning and transmission electron microscopy studies. This provided crucial insights into the ultrastructural composition of cortical cytoplasmic bridges, enriched with endoplasmic reticulum, fragmented vacuoles and mitochondria around the nucleus, opening new perspectives for understanding their potential role. These massive ultrastructural changes are unique to cells committed to rhizobia infection, highlighting the need for rhizobia in close proximity to induce them using likely microbial signals.
In Medicago root cortex, a fine tuning of calcium spiking during infection was reported1, but how this relates to infection priming or cytoplasmic remodelling was unknown. Guillory et al. 20243 explored this question using an original, co-dynamic approach based on live monitoring calcium spiking and cytoplasmic remodelling simultaneously. We chose the red fluorescent calcium sensor NR-GECO1, ideal for co-dynamic studies with green fluorescent cytoplasmic markers. Furthermore, NR-GECO1’s ability to reliably measure symbiotic signalling similar to a ratiometric calcium probe but with greater sensitivity3 made it ideal for live imaging in less accessible root cortex. MtAnn1 appeared to be an ideal cytoplasmic marker for labelling cytoplasmic rearrangements, as it shows rhizobia-infection related expression and encodes a cytoplasmic calcium-binding annexin protein potentially involved in this process.
Established methods for monitoring rhizobial root hair infection in vivo were essential, as only growth-active infection sites form cytoplasmic bridges. Monitoring the in vivo co-dynamics of NR-GECO1 and MtAnn1 over time in this system was also critical to show the close relationship between calcium spiking and MtAnn1 dynamics. This would not be possible with fixed materials. Using the sunn mutant, with a higher number of rhizobia entry points, was also crucial to analyse these events in more sites. An important finding was that only cortical cells in direct contact with infected hairs showed reprogramming (calcium oscillations and MtAnn1 accumulation), suggesting active signalling from infected hairs to neighbouring primed cells. This open interesting perspectives to study what is transmitted and how. Importantly, using the NR-GECO1-MtAnn1 module in different mutant backgrounds revealed that symbiotic signalling involves distinct calcium spiking signatures, set in different phases and tissues under different genetic dependencies (depending or not on the central calcium decoder DMI3 or the master transcriptional regulator NIN).
Finally, plant and animal annexins are involved in the regulation of several calcium-regulated cellular mechanisms. Phenotypic analyses of MtAnn1-tagged mutants or RNAi roots allowed us to correlate MtAnn1 with the regulation of calcium amplitude during rhizobial symbiosis and to establish a link with the creation of a cytoplasmic environment suitable for rhizobial infection. The work on this material also demonstrated once again the power of high-resolution microscopy approaches to shed light on fine modifications that cannot be detected by macroscopic analysis. Finally, phylogenetic analysis based on a large number of plant species has driven functional studies of MtAnn1 in AM endosymbiosis, which together strengthen the ancient recruitment of MtAnn1 for root endosymbioses.
References
1 de Carvalho-Niebel, F., Fournier, J., Becker, A. & Marín Arancibia, M. Cellular insights into legume root infection by rhizobia. Curr Opin Plant Biol 81, 102597 (2024). https://doi.org/10.1016/j.pbi.2024.102597
2 Gutjahr, C. & Parniske, M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 29, 593-617 (2013). https://doi.org/10.1146/annurev-cellbio-101512-122413
3 Guillory, A. et al. Annexin- and calcium-regulated priming of legume root cells for endosymbiotic infection. Nat Commun 15, 10639 (2024). https://doi.org/10.1038/s41467-024-55067-3




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in