How MDA Phages Select Hyperadhesive Variants in Neisseria meningitidis?
Published in Microbiology and Cell & Molecular Biology
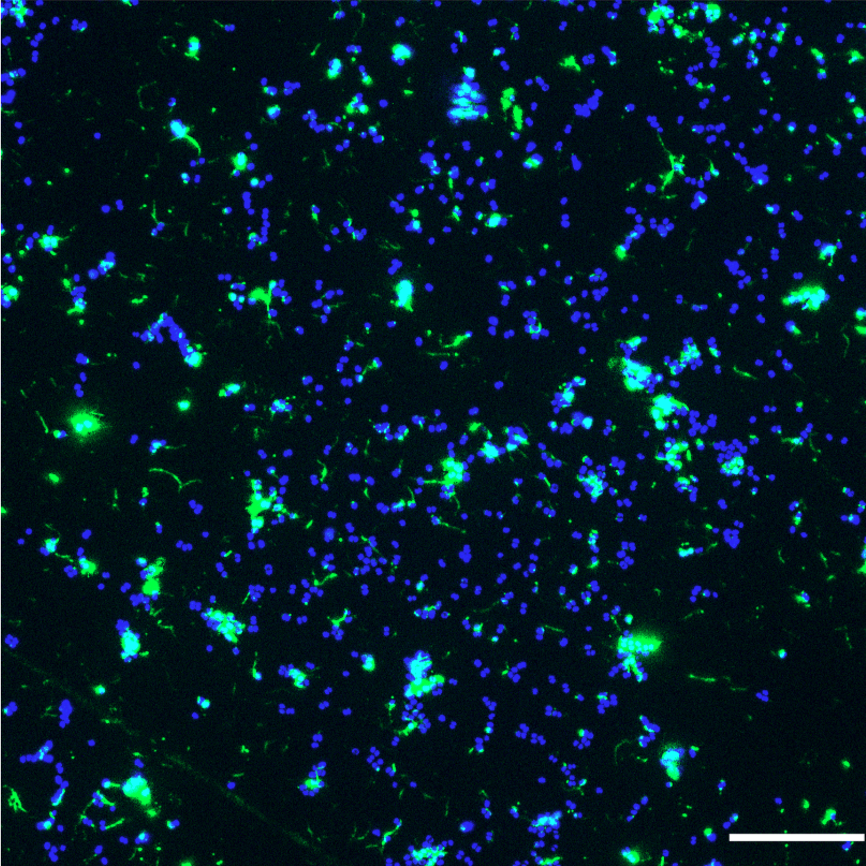
Background
Filamentous phages are non-lytic bacterial viruses that can integrate into bacterial genomes, often modifying host fitness. Unlike well-studied viruses such as Caudovirales, which have a tail and a head, filamentous phages such as CTXΦ in Vibrio cholerae and Pf4 in Pseudomonas aeruginosa have a filamentous shape, a worm-like chain (long, thin, and flexible), and contribute to biofilm formation, immune evasion and virulence of theirs hosts.
In N. meningitidis, the MDA phage is associated with increased biofilm formation and invasiveness.
N. meningitidis possesses several other virulence factors that are necessary for its virulence, such as the capsule (to avoid complement attack in the blood) and type IV pili. These pili are dynamic surface filaments essential in numerous bacterial functions including bacterial interaction with human cells, DNA uptake, twitching motility and bacterial-bacterial interactions. However, although these pili are essential, their main protein (PilE, whose assembly forms the pilus fiber) is subject to antigenic variation and is therefore extremely variable.
Objectives
We aimed to understand how the phage MDA interacts with type IV pili of N. meningitidis and how this interaction affects phage propagation and bacterial colonisation of eukaryotic cells.
Key Findings
-
Phage Entry Requires Retractable Type IV Pili
- Phage transduction was impaired in mutants lacking PilT (retraction motor) or PilE (major pilin protein).
- Other pilus-associated proteins, especially those at the tip (PilC1, PilC2, PilH, PillI, PilJ, and PilK), were not essential for phage entry.
-
The MDA phage Targets Pili Along Their Length, Not the Tip
- Unlike other filamentous phages that bind to the pilus tip, MDAΦ binds along the entire pilus fiber.
-
Antigenic Variation Drives Phage Susceptibility
- Class I pilE genes (major pilin) undergo antigenic variation via recombination with silent pilS
- MDAΦ preferentially infects bacteria expressing specific PilE variants, which are more positively charged.
- Even in strains engineered to express a single pilE variant, antigenic variation still occurred, allowing selection of phage-susceptible clones.
-
Phage Binding Correlates with Pili Charge
- This binding is preferential to pili formed by positively charged variants of the major pilin protein PilE.
- The major phage coat protein ORF4 is negatively charged, suggesting electrostatic attraction facilitates binding between the phagic capsid and the pili.
-
The phagic protein ORF6 Is Not Involved in Pili Binding
- ORF6, a protein encoded by the phage and described as an adsorption protein, does not interact with pilus tip proteins.
- Phage binding occurred even in phages lacking ORF6.
-
Adhesion and Phage Infection Select the Same PilE Variants
- Adhesion to human cells and phage transduction both enriched for bacteria expressing a precise PilE variant positively charged.
- MDAΦ preferentially infects bacteria expressing specific PilE variants, which are more adhesive.
- This suggests a dual selection mechanism where hyperadhesive bacteria are also more susceptible to phage infection, promoting colonization.
Implications
This study reveals a novel mechanism by which a filamentous phage promotes bacterial colonization: by selectively infecting and amplifying hyperadhesive variants of N. meningitidis. This dual role of pili in adhesion and phage susceptibility links phage biology directly to bacterial virulence and population dynamics.
Conclusion
MDAΦ does not follow the classical model of pilus-tip binding seen in other filamentous phages. Instead, it binds along the pilus fiber, favouring positively charged PilE variants that enhance bacterial adhesion. This interaction promotes the selection of hyperadhesive bacteria, potentially increasing the invasiveness and transmissibility of N. meningitidis.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in