How we stumbled upon aromatic lactic acids in infant stool samples
Published in Microbiology

This is the story behind: 'Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut'
It all started in a basement at the National Food Institute, Technical University of Denmark, outside Copenhagen on a Winther morning in 2016. This morning we stumbled upon the fact that infant stool samples contained a thousand fold higher levels of indolelactic acid compared to stool samples of adults. It was basically a coincidence that we observed this in a few infant stool samples during the development of a quantitative liquid chromatography mass spectrometry (LC-MS) method together with Henrik Frandsen, an experienced and outstanding chemist.
Together with my close colleague and friend Martin Laursen, who at that time was a PhD student studying how dietary changes affect the infant gut microbiota in the Danish SKOT cohort (in collaboration with Mads Vendelbo Lind, Kim F. Michaelsen, and Christian Mølgaard), I (Henrik Roager) started questioning the remarkably high levels of indolelactic acid in the infant stool samples. We decided to analyse more samples from the cohort, motivated by the idea that indolelactic acid may be linked to both diet and gut microbiota in early life. We quickly realized that the abundance of indolelactic acid was associated with the abundance of Bifidobacterium in the stool samples and therefore hypothesized that Bifidobacterium species were producing indolelactic acid in the infant gut.
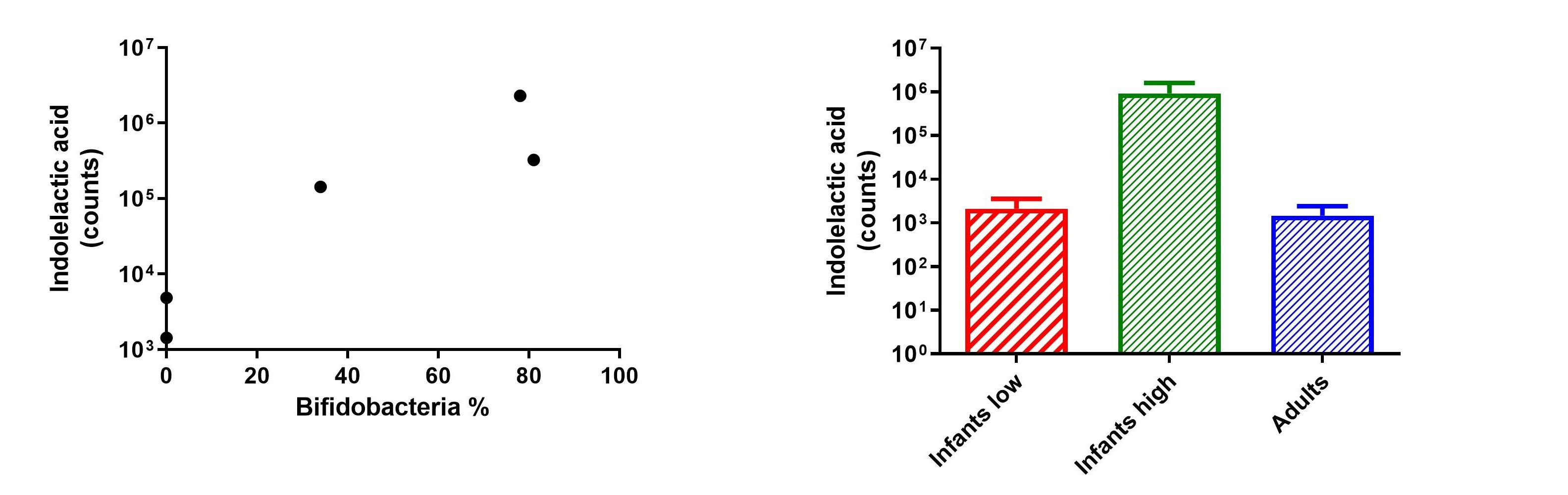
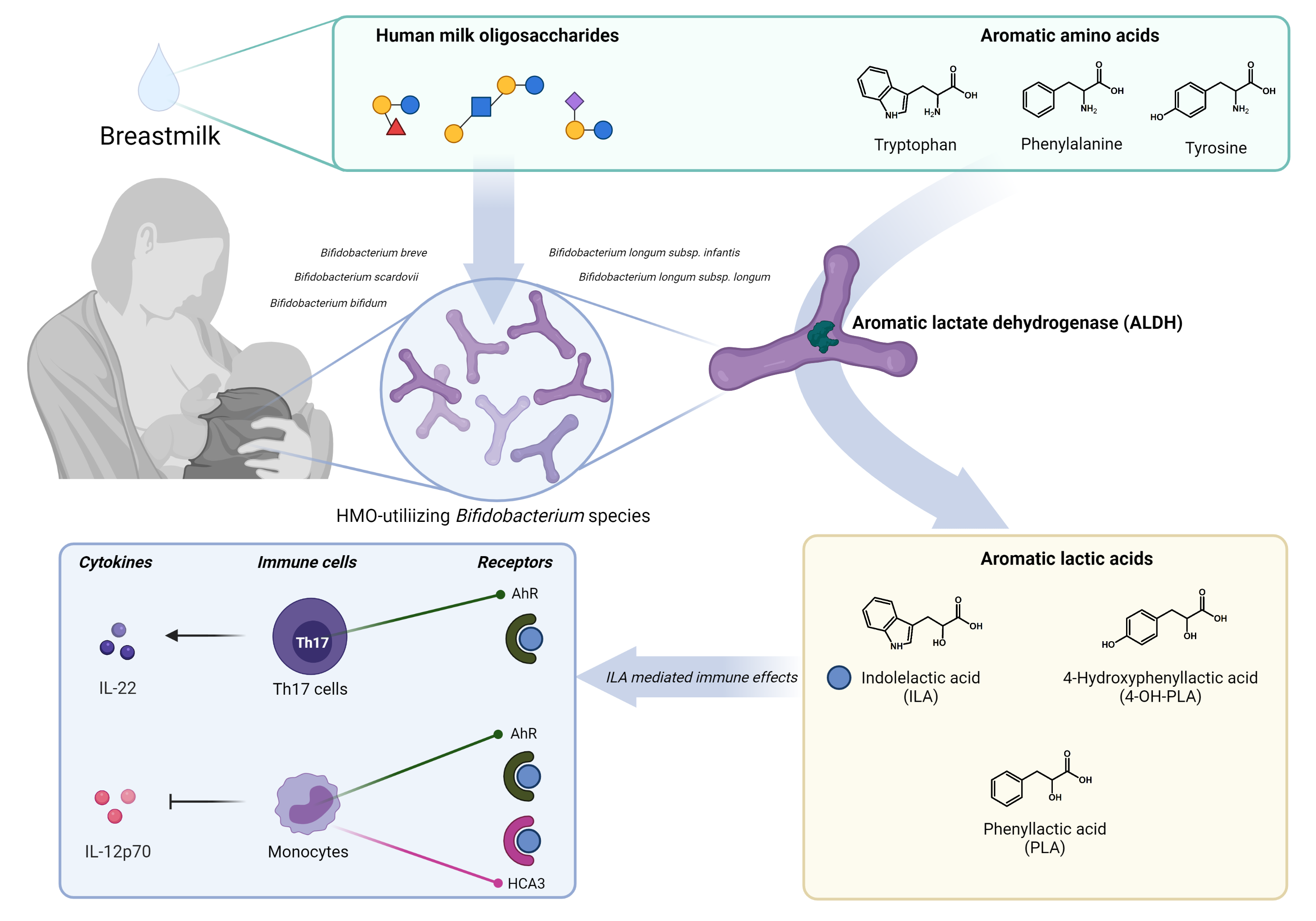
In the lab, we decided to grow representative strains of the various Bifidobacterium species found in the infant cohort. Indeed, Bifidobacterium species were able to produce indolelactic acid (and the other aromatic lactic acid, phenyllactic acid and 4-hydroxyphenyllactic acid), however not all species to the same degree. There was a stunning link between the production level of the aromatic lactic acids and the ability of a Bifidobacterium species to utilize human milk oligosaccharides (HMOs), key microbiota-modulating components of breastmilk. Therefore, some features of the specific HMO-degrading Bifidobacterium species promoted by breastfeeding rendered them prominent producers of the aromatic lactic acids.

We learned from papers on Lactobacillus strains, that an aromatic lactate dehydrogenase could be responsible for the conversion of aromatic pyruvates into aromatic lactic acids1. We hypothesized that the same could be the case in Bifidobacterium. Therefore, we searched for all available lactate dehydrogenase genes in Bifidobacterium species and found that the breastmilk-promoted (HMO-degrading) species producing high levels of all three aromatic lactic acids in vitro were characterized by having an additional lactate dehydrogenase gene besides the lactate dehydrogenase gene responsible for the conversion of pyruvate into lactate. When cloning the gene into an E. coli (under excellent supervision of Martin I. Bahl), we could show that the gene (hereafter denoted aldh) was responsible for the production of aromatic lactic acids. We had the feeling that we were on to something and what started as a random discovery had suddenly turned into a promising side project with the full support from our nearest colleagues including Prof. Tine Licht.
Our excitement was fueled by the fact that during these years, several tryptophan-derived metabolites were reported to modulate immune responses2. We hoped we could show the same for indolelactic acid. Therefore, we decided to go all in in order to bring the project to a whole new level. Although it remained as a side project in terms of time and funding the whole time, we were very fortunate to obtain funding from several small Danish funds who saw the potential.

Furthermore, we were fortunate to get in contact with Mikiyasu Sakanaka, an expert in genetic manipulation within Bifidobacterium. He and Prof. Takane Katayama (Ishikawa Prefectural University, Japan) generated an aldh knockout Bifidobacterium longum strain, which we later introduced to germ-free mice and demonstrated that the gene was in fact responsible for the production of aromatic lactic acids in vivo. Mikiyasu and Takane also enzymatically characterized the aromatic lactate dehydrogenase and confirmed its preference for aromatic pyruvates over pyruvate.
Inspired by our own experiences with babies at home, Martin and I decided to collect (with the help of the parents) stool samples from birth until 6 months of age from 25 Danish infants – thanks to all volunteers! The analyses of the stool samples provided insights into the early dynamics of aromatic lactic acids in the infant gut and provided evidence of the close connection between the abundance of Bifidobacterium species and the levels of aromatic lactic acids within individuals over time. In addition, our friend Janne Marie Moll replicated the findings in publically available data from another infant cohort3 and underscored the strong link to breastfeeding.

Our great collaborators Ceyda Pekmez and Prof. Lars Dragsted at Department of Nutrition, Exercise and Sports, University of Copenhagen, measured aromatic lactic acids in urine of the SKOT infants and demonstrated that the aromatic lactic acids are absorbed from the gut into the circulation in infants, suggesting that these metabolites may have systemic affects.
Finally, we involved some outstanding molecular biologists and immunologists (Nicole von Burg, Daniel Andersen, Urs Mörbe, Aymeric Rivollier, Anne Marie Vinggaard, Susanne Brix, and William Agace) who we admire a lot. They were key in demonstrating that indolelactic acid can modulate immune function via the aryl hydrocarbon receptor (AHR) and the hydroxycarboxylic acid receptor 3 (HCA3).

What started as a coincidence, ended up as an elegant story showing that Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut due to presence of an aromatic lactate dehydrogenase, and that these metabolites have the potential to affect immune function in early life via AHR and HCA3. We think this opens up for new avenues to be explored and we are truly excited about how the scientific community will move this field of research forward.
We have learnt a lot through the years and could not have done this without the help from the parents helping with collecting stool samples and a massive team effort.
Cover photo PublicDomainPictures at PixaBay
References:
- Li, X., Jiang, B., Pan, B., Mu, W. & Zhang, T. Purification and partial characterization of Lactobacillus species SK007 lactate dehydrogenase (LDH) catalyzing phenylpyruvic acid (PPA) conversion into phenyllactic acid (PLA). J. Agric. Food Chem. 56, 2392–2399 (2008).
- Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294 (2018).
- Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in