Hydrolysis-Engineered Robust Porous Micron Silicon Anode for High-Energy Lithium-Ion Batteries
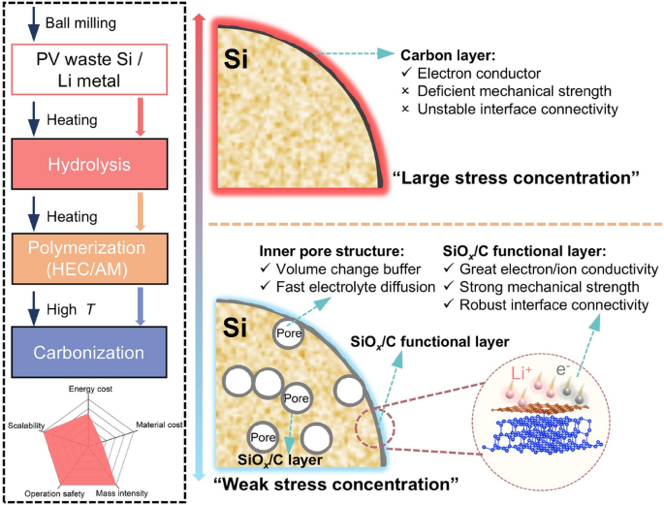
Silicon anodes could catapult lithium-ion energy density past 400 Wh kg-1, but every 300 % volume swing pulverizes the electrode and drains the electrolyte. Nano-porous designs tame swelling yet sacrifice tap density and calendar life; conventional carbon shells crack under industrial calendering. As reported in Nano-Micro Letters, a South China University of Technology team led by Prof. Longtao Ma and Prof. Liuzhang Ouyang has unveiled a “breathing” micron-Si anode that turns waste photovoltaic chips into 901 mAh g-1 after 500 cycles at 1 A g-1, 850 mAh g-1 at 8 A g-1, and a stack volumetric energy density of 896 Wh L-1—all without a drop of HF or toxic siloxane.
Why This Micron-Si Matters
- Industrial Durability: 500 deep cycles at 1 A g-1 with 60 % retention—no fragile nano-scaffolding.
- Fast-Charge Ready: sustains 8 A g-1(>10 C) and recovers 1,677 mAh g-1 when load relaxes.
- Mechanical Resilience: electrode thickness expansion held to 45 %—half that of carbon-coated Si.
- Green & Scalable: starts from waste PV Si chips, skips corrosive etchants, and meets cost targets for 300 Wh kg-1 pouch cells.
Inside the “Breathing” Architecture
1. Hydrolysis-Driven Inside-Out Functionalization
- Mild water hydrolysis(55 °C) selectively etches micron-Si, opening 5–10 nm pores while forming a thin SiO2 sol layer on every surface—inner pores and outer shell alike.
- One-pot polymerization of hydroxyethyl cellulose + acrylamide infiltrates pores, cross-links, and carbonizes into 7 nm nitrogen-doped SiOx/C skins chemically grafted to Si.
- Single Ar calcination (700 °C) completes the dual-layer coating—no HF, no Mg reduction, no siloxane precursors.
2. Stress-Relief & Ion-Highway Design
- Finite-element modeling shows full-lithiation von Mises stress plummets from 47 GPa (pristine Si) to 9.9 GPa for dual-coated porous Si; pore-edge stress drops from 29 to 13 GPa.
- In-situ LixSiOy formation: first-cycle lithiation converts surface SiOx into fast-ion conductors, lowering diffusion barriers while carbon maintains electron wiring.
- Elastic modulus tuned to 1.1 GPa balances flexibility and adhesion, preventing shell detachment during 300 % expansion.
3. Electrochemical Milestones
- Half-cell: 1,485 mAh g-1 initial charge at 1 A g-1; 901 mAh g-1 after 500 cycles—outperforming most micron-Si literature.
- Rate capability: 1,123 mAh g-1 at 5 A g⁻¹ and 850 mAh g-1 at 8 A g-1—enabled by 83 % pseudocapacitive contribution at 1 mV s-1.
- Pouch cell (2.1 mAh cm-2 anode | 18.75 mg cm-2 NCM811 cathode): retains 2.09 mAh cm-2 over 100 cycles at 1 C—equivalent to 300 Wh kg-1 full-cell projection.
Mechanistic Insights: Pore + Skin Synergy
1. Operando Raman & XPS
- Raman mapping during first lithiation reveals inorganic-rich SEI (LiF/LixSiOy) with 50 % lower organic carbonate residues versus bare Si—translating to charge-transfer resistance drop from 49 Ω to 8 Ω.
- XPS depth profiling confirms Si–O–C chemical grafting and uniform N-doped carbon network that endures 500 cycles without delamination.
2. Post-mortem & TOF-SIMS
- SEM/TOF-SIMS after 100 cycles: minimal crack propagation and 70 % lower CH2- fragments, confirming suppressed electrolyte decomposition and mechanical integrity.
Toward Gigawatt-Hour Production
1. Green Manufacturing
- Feedstock: waste photovoltaic Si chips (~US$2 kg-1) ball-milled to 2–5 μm.
- Process: water hydrolysis → polymer infiltration → single-step calcination; energy intensity 30 % lower than CVD or Mg-reduction routes.
- Safety: no HF, no siloxane fumes; waste-water contains only neutral SiO2 colloids—readily filtered.
2. Roadmap
- Pilot line (2026): roll-to-roll slot-die coating of 10 kg batches; target 2.5 mAh cm-2 areal loading for 300 Wh kg-1 pouch cells.
- Next-gen targets: Si–C composite (20 wt % carbon) for 95 % retention at 500 cycles; solid-state interface with sulfide electrolytes for 400 Wh kg-1 prototypes.
- End-of-life: anode material directly recyclable into new hydrolysis feedstock—closing the silicon loop.
With roll-to-roll pilots already spinning out 10 kg batches and a clear path to 400 Wh kg-1 solid-state cells, this hydrolysis blueprint is poised to migrate from lab benches to gigawatt-hour gigafactories—delivering cheaper, faster-charging, and longer-lasting batteries built from yesterday’s solar waste.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in