Immune Engineering - Development of Immuno-Materials
Published in Bioengineering & Biotechnology

The human immune system plays an integral role in health and disease, and its dysregulation drives the aetiology of cancer1, autoimmune diseases2 and cardiovascular disease3 (inflammatory hypothesis in atherosclerosis). The focus here is on a relatively new niche of biomaterials that combine materials science with the known molecular basis of immunity in health and disease, to form immuno-materials. Immuno materials are developed for immunotherapy or immunodiagnostics, with the material design principles focused on the intended biological functionality. In immunotherapy, for instance, biomaterials are designed to induce targeted activation/suppression of an immune response. In immunodiagnostics, the material is designed to interrogate cellular responses of the immune system. The cells of the immune system thereby dictate the rules to create biologically-inspired materials for intervention in immunity.
Based on biomimetic design, ensuing material systems already have broad applications in medicine (Fig 1), which include;
- Detection of the immune response,
- Imaging immune cell trafficking,
- Targeted delivery of modulating agents,
- Nanoparticle-based vaccines, and the more recent, personalized vaccine development4,5.
The aim to intervene and regulate the immune system during immune deficiency is a feasible research strategy. A variety of techniques are in development to manipulate immune cells ex vivo and in the human body. At present, Immuno-materials are an active research focus in development at the Wyss Institute (Video 1); mainly evolving from previously established technologies for programmable nanomaterials6,7.
Video 1: Wyss Focus: Immuno-Materials (Uploaded by Harvard University, May 2017).
As the number of strategies to re-awaken T-cells via immunotherapy increase, tumor cells also rapidly evolve, so that only a subset of tumors therapeutically regress. In light of this, more recent work has focused on the development of mathematical models that predict the efficacy of anti-tumor immune responses in any given patient8,9.
While the development of new immuno-materials for new therapies are underway, this article recaps established concepts underlying the strategy. For brevity, the focus is on the fabrication of biodegradable materials for adaptive immunotherapy6,10,11.
Biologically-Inspired Design for Artificial Antigen-Presentation
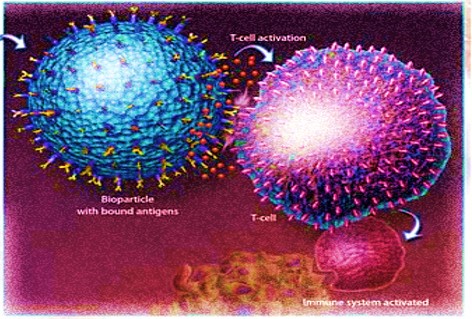
Briefly, T cells in the immune system are specialized to identify and eliminate infected cells and cancer cells that pose a threat to humans. Most cell surfaces present peptide fragments known as antigens, generated from intracellular proteins. Healthy T cells scan the body in search of cells displaying antigens from infectious organisms, or neoantigens (new antigens) expressed due to cancerous mutations. Professional antigen-presenting cells known as dendritic cells usually interface with T-cells for subsequent T-cell activation against the specific antigen presented12. During tumorigenesis, this surveillance system is hijacked to suppress the immune response.
In biologically-inspired design, biodegradable particles can be engineered to mimic native dendritic cells and activate the T-cells, via artificial antigen presentation (Fig 2)11.
To accurately engineer artificial antigen presenting cells (aAPCs) that normally facilitate T-cell activation; biological recognition signals (peptide-major histocompatibility complex-MHC) and stimulatory signals should be incorporated to the biodegradable platform. Particles can be fabricated in the micro/nanometer scale from poly(lactide-co-glycolide) (PLGA) polymers, whose safety in humans has been ensured11. Based on the high-affinity binding between avidin and biotin; avidin was immobilized on particle surfaces during synthesis, for attachment of biotinylated MHC dimers and antibodies, for recognition and co-stimulation of T cells. The particles were then characterized with scanning electron microscopy (SEM) and qualitatively visualized to observe stable interactions with T cells (Fig 3 a-c).
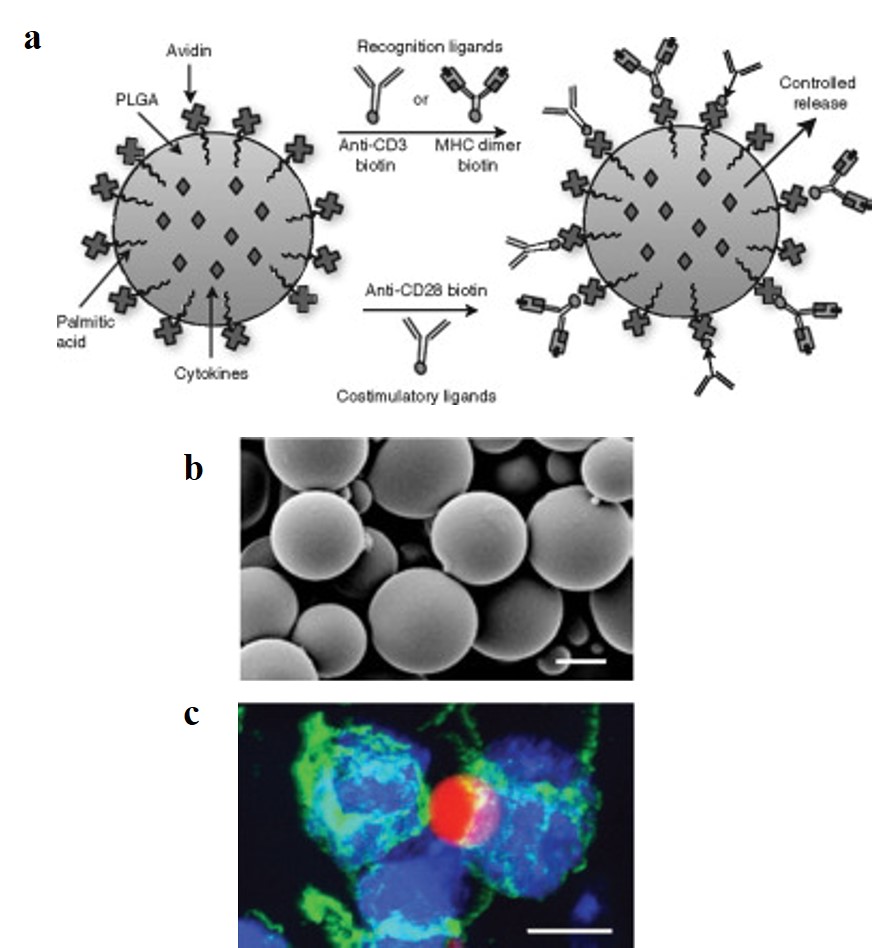
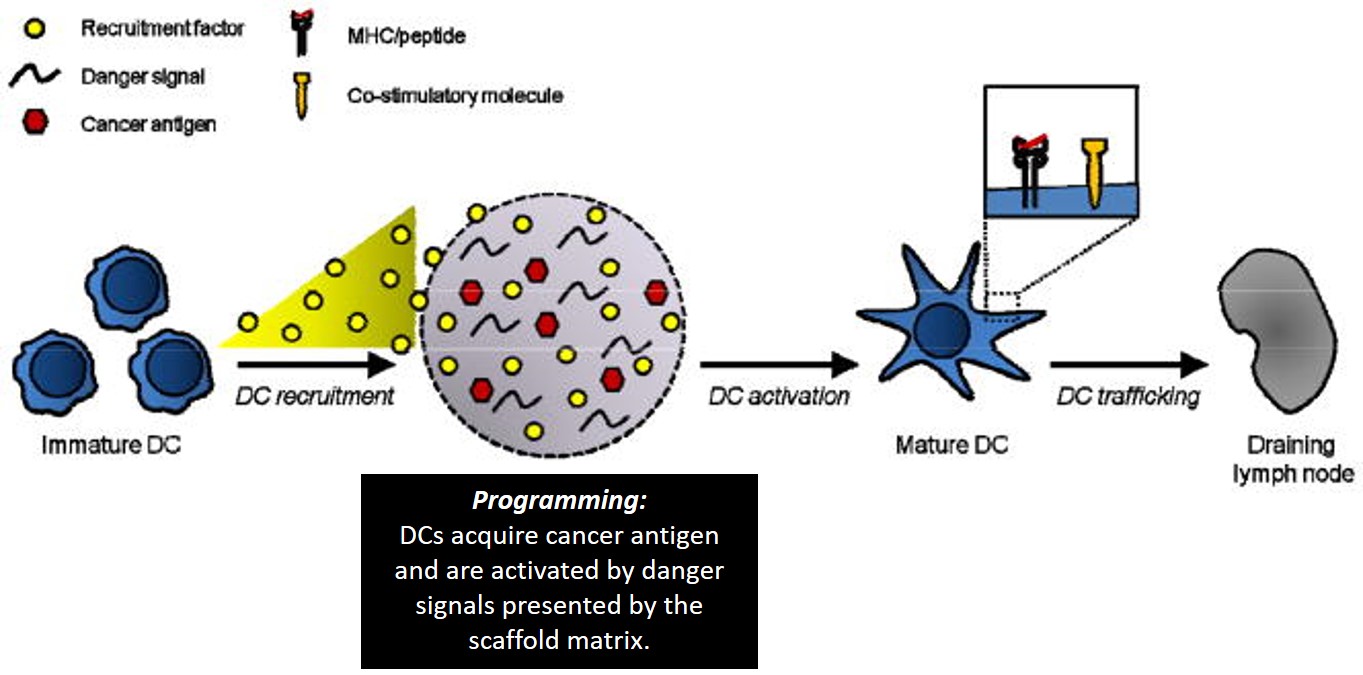
These biodegradable aAPCs represent pioneering work in immunomaterials for therapeutic and investigative applications. Additionally, microenvironments can be engineered to program dendritic cells (DCs) to initiate a T cell response; as therapeutic cancer vaccines. This was investigated with highly porous scaffolds, similarly fabricated from biodegradable copolymer PLGA (poly[lactide-co-glycolide]), integrated with bioactive molecules to recruit DCs. Inside the engineered microenvironment or matrix, DCs acquire cancer antigens, to present acquired peptides on cell surface MHC molecules, initiating a T cell response in vivo thereafter (Fig 4). A 90% survival rate was achieved in mice with melanoma via melanoma-antigen-specific T-cell generation, indicating a promising cancer vaccine6. This ground breaking potential is now being tested in clinical trials as a personalized cancer vaccine with human melanoma patients5.
These studies have demonstrated a powerful new application of polymeric biomaterials via molecular engineering and re-programming (or programming). Further studies have also explored alternative materials including nucleic acids for personalized immunotherapy and diagnostics4. Researchers hope to integrate such immune-related efforts from other focus areas, alongside the diverse pioneering techniques, to invent next-generation immuno-materials for broader utility.
Poster Image: Scanning electron microscope image of autoimmune T cell-trapping biomaterials, developed at the Wyss Institute.
References:
- Coussens, L.M. & Werb, Z. Inflammation and cancer. Nature 420, 860-867 (2002).
- Straub, R.H. & Schradin, C. Chronic inflammatory systemic diseases. An evolutionary trade-off between acutely beneficial but chronically harmful programs. Evolution, Medicine, and Public Health 2016, 37-51 (2016).
- Harrington, R.A. Targeting Inflammation in Coronary Artery Disease. New England Journal of Medicine 377, 1197-1198 (2017).
- Sahin, U., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222-226 (2017).
- Ott, P.A., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217 (2017).
- Ali, O.A., Huebsch, N., Cao, L., Dranoff, G. & Mooney, D.J. Infection-mimicking materials to program dendritic cells in situ. Nature Materials 8, 151 (2009).
- Cheung, A.S. & Mooney, D.J. Engineered Materials for Cancer Immunotherapy. Nano today 10, 511-531 (2015).
- Łuksza, M., et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517 (2017).
- Balachandran, V.P., et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512 (2017).
- Steenblock, E.R., Wrzesinski, S.H., Flavell, R.A. & Fahmy, T.M. Antigen presentation on artificial acellular substrates: modular systems for flexible, adaptable immunotherapy. Expert Opinion on Biological Therapy 9, 451-464 (2009).
- Steenblock, E.R. & Fahmy, T.M. A Comprehensive Platform for Ex Vivo T-cell Expansion Based on Biodegradable Polymeric Artificial Antigen-presenting Cells. Molecular Therapy 16, 765-772 (2008).
- Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245 (1998).
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in