Investigating the underlying molecular mechanisms of Fc receptors for immune engineering
Published in Bioengineering & Biotechnology, Materials, and Protocols & Methods
Fragment crystallizable receptors (FcRs) for IgG known as FcγR (FcgammaR) mediate a range of effects, including the clearance of immune complexes, antibody-dependent cellular cytotoxicity (ADCC), and the phagocytosis of renal calculi during kidney stone formation [Guyre 1998, Fitzer-Attas 2000, Sips 2016]. These receptors can also enhance antigen presentation and the secretion of reactive oxygen intermediates, and phagocytosis of antibody-coated pathogens. In my most recent research work, I noted the transcriptomics-based upregulation of FcγR-phagosomes in renal kidney patients [Jeewandara 2023, ref 4]. These phagosomes are triggered in response to an increased buildup of calcium during pathological biomineralization at the renal papillary tip, to activate circulating or kidney resident anti-inflammatory M2 macrophages to engulf and clear the calculi [Khan 2021]. Most FcγRI are constitutively expressed in macrophages and monocytes [Nimmerjahn 2008].
In this post, my research interests in immune-engineering resurface, and intersect with a brief outlook at basic science and bioengineering, akin to my previous articles on immune-materials as also published on Nature Portfolio that combined immunology and materials science to describe an aspect of immunomodulation with immune-materials for therapeutic intervention (Figure 1). This post begins with a key focus on macrophages and broadens to the varied Fc receptors of human leukocytes and to their soluble forms, with relevance across several pathologies, to then explore bioengineering strategies for translational immune engineering and the development of antibody therapies for precision medicine.
Figure 1: A brief recap of my research interests in immune engineering through the years to develop immune materials for immunotherapy and immunodiagnostics [Jeewandara T.M., 2017].
Inflammation is a common denominator of disease including chronic kidney disease, renal calcification, auto-immune diseases, allergies, cancer, HIV, and cardiorenal disease (based on the inflammatory hypothesis of atherosclerosis) to name a few [Sips 2016, Harrington 2017]. During the progression of complex diseases such as nephrolithiasis, M2-like macrophages typically suppress inflammation through the phagocytosis of renal calculi, to attenuate kidney stone formation, and similarly exert their roles in other inflammatory pathologies too. Conversely, pro-inflammatory M1 macrophages-facilitate calcium oxalate-based crystal deposition during early renal biomineralization [He 2022, Khan 2021].
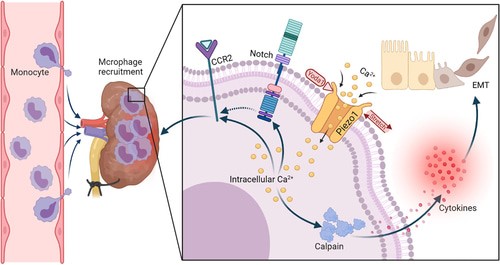
Figure 2: A schematic representation of the renal microenvironment that includes the recruitment of macrophages during renal fibrosis and the activation of mechanosensory ion channels to elicit a pathological response [He 2022].
The M2 macrophages also enact anti-inflammatory actions across a variety of disorders to circumvent tumor formation, infection, and obesity [Vogelpoel 2014]. The common method of human macrophage M1 and M2 formation in the lab to understand their functionality is facilitated via the differentiation from monocytes or peripheral blood mononuclear cells in culture, through either granulocyte-macrophage stimulating factors (GM-CSF) or macrophage stimulating factors (M-CSF), respectively [Jeewandara 2023, Vogelpoel 2014]. The capacity to regulate M2 polarization in the lab has scope for its therapeutic value in renal medicine, specifically due to its influence in renal calcification.
For instance, using methods of molecular engineering the M2 macrophages can be therapeutically upregulated, as opposed to M1 macrophages, to reduce the localized aggregation of birefringent crystals of calcium oxalate in the renal papillae of the kidney via phagocytosis. With capacity to experimentally emulate its pre-clinical impact initially with biopsy-derived tissue of stone-formers cultured in a microphysiological kidney-on-a-chip environment in vitro [Khan 2021, Khan 2014, Jeewandara 2023]. However, the therapeutic effects of such immunomodulating receptors are not always consistent, since they vary in their pro- or anti-inflammatory effects across different pathologies (Figure 3).
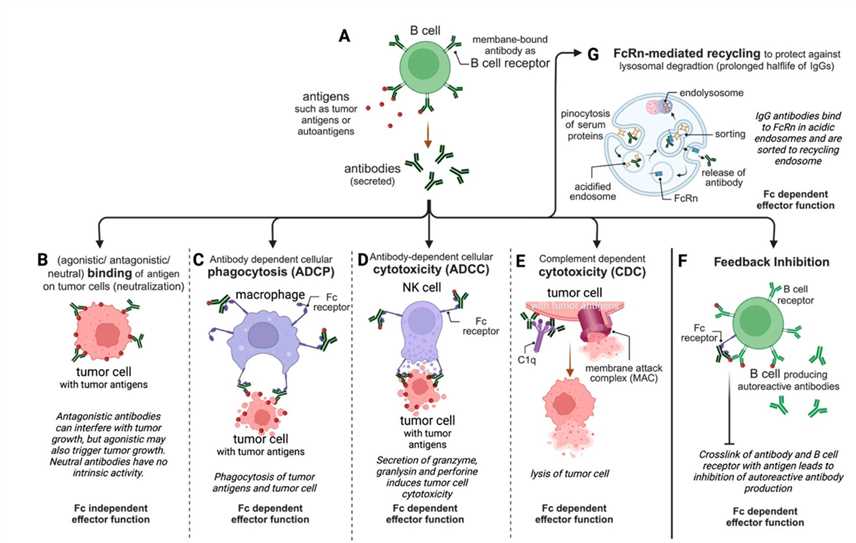
Figure 3: A schematic representation of the important therapeutic antibodies currently in use [Creative Biolabs].
Getting to know the multifaceted role of FcγRs during inflammatory diseases.
Beyond their pro or anti-inflammatory role of activating or preventing infection, the Fc receptor functions are also relevant for post-infection control and clearance of viral infections such as Ebola and influenza as well as the spontaneous durable regulation of the viral load in HIV infections [Sips 2016]. As a result, immunologists and bioengineers aim to understand the Fc receptor involvement across inflammatory diseases, to explore their varied mechanisms-of-action, and develop new therapies [Sips 2016, Mkaddem 2019]. Although the capacity of M2 macrophages to suppress inflammation across various disorders (including nephrolithiasis) is known, its impact on a multitude of other diseases remains largely undefined. Details of the various human Fc receptors, their expression, function, and mediation of a variety of pathologies are listed in Table 1 [Mkaddem 2019].
For example, in rheumatoid arthritis, M2 macrophages promote inflammation instead of its suppression (Figure 4) [Vogelpoel 2014, Hoepel 2019]. Study outcomes have nevertheless shown the capacity to regulate therapeutic complications of M2 macrophages in rheumatoid arthritis by targeting the FcγR-toll-like receptor crosstalk for focused treatment, to reduce disease-related inflammation by restoring the anti-inflammatory function of M2 macrophages [Vogelpoel 2014]. Since M2 macrophages upregulated the tumor necrosis factors alpha and interleukins (IL) 1β and IL6 for involvement in rheumatoid arthritis pathology [McInnes 2011]. The inhibition of FcγR function on M2 macrophages can act as a potential therapeutic target to block the production of pro-inflammatory cytokines, while leaving interleukin 10 (anti-inflammatory cytokine) production intact [Vogelpoel 2014].
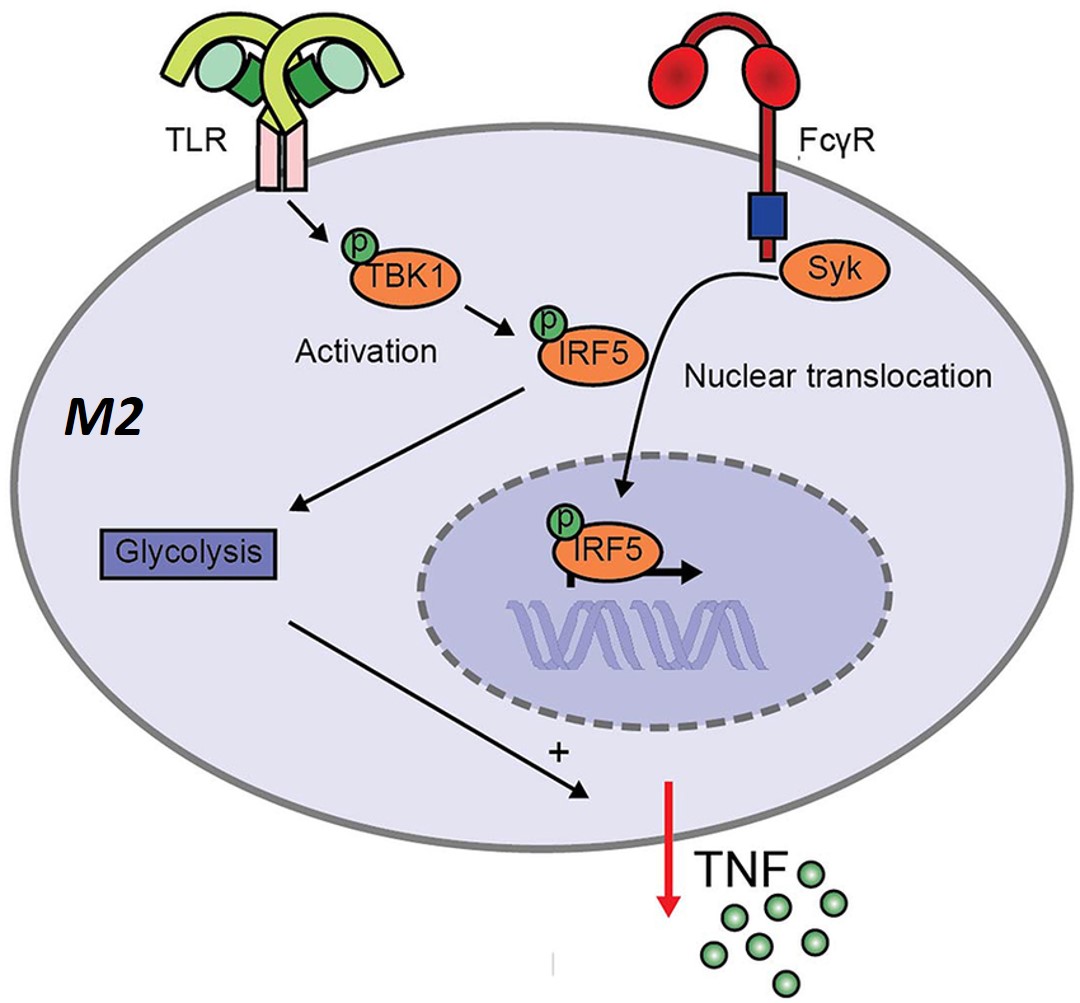
Figure 4: Although M2-polarized macrophages are generally classified as anti-inflammatory, the complexed IgG and TLR co-stimulation in infections or rheumatoid arthritis elicit an inflammatory response in M2 macrophages [Vogelpoel 2014, Hoepel 2019].
Many effects that are induced by activating the FcγRs, including FcγRI and IIa, depend on signaling via the spleen tyrosine kinase (syk) – a key therapeutic target [Nimmerjahn 2008]. Incidentally, the influence of syk (spleen tyrosine kinase) for FcγR-toll-like receptor crosstalk in M2 macrophages can be blocked by using R406 (also known as tamatinib) – a kinase inhibitor. This molecular medicine compound is presently in use to treat rheumatoid arthritis patients [Weinblatt 2010], and it also appears to restore the anti-inflammatory profile of M2 macrophages, alongside a minimal secretion of pro-inflammatory cytokines.
The outcomes further delineated proinflammatory cytokines induced via FcγR-toll-like receptor crosstalk to be syk-dependent, to show how FcγR-induced inflammation via M2 macrophages can be therapeutically counteracted via kinase inhibition. The multifaceted functionality of Fc receptors for IgG known as FcgammaR can also be investigated and translated to form new therapeutic strategies to treat human autoimmune diseases such as lupus erythematosus and rheumatoid arthritis, as well as other infectious, and malignant diseases [Mkaddem 2019].
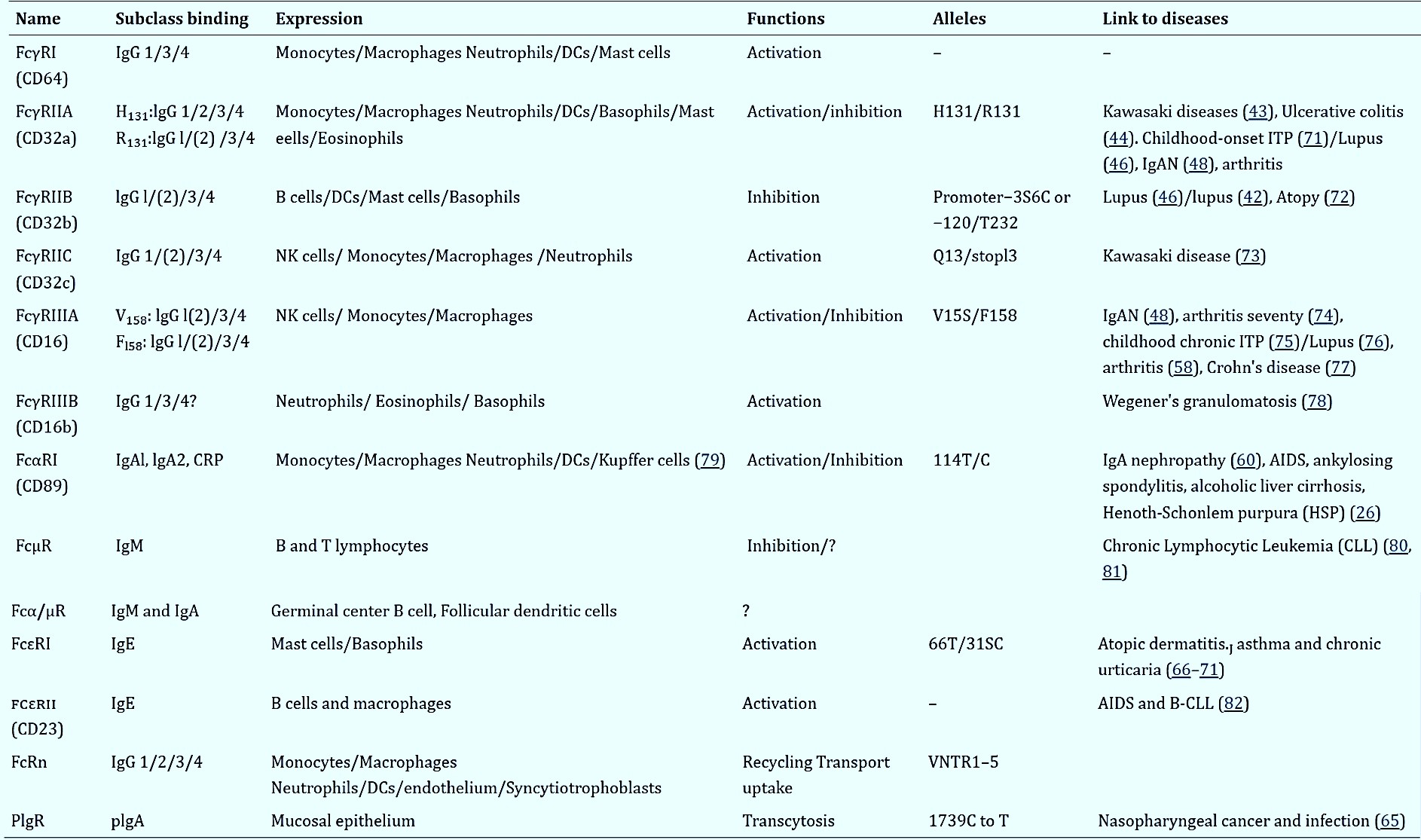
Table 1: (Zoom in for clarity). Human FcRs: their expression, function and allotypes. From left to right columns: names, function, alleles that include amino acid variations in immunoglobulin domains and the transmembrane domain, cellular expression of FcRs, and diseases linked to alleles and CNVs (reference numbers are shown), and binding abilities of IgG subclasses to each FcR allele. IgAN, IgA nephropathy; CLL, chronic lymphocytic Leukemia; FcRn, neonatal Fc receptor; AIDS, acquired immune deficiency syndrome; PIgR, Polymeric immunoglobulin receptor; DCs, dendritic cells. [Mkaddem 2019].
The structure and mechanism-of-action of FcRs and their cellular expression
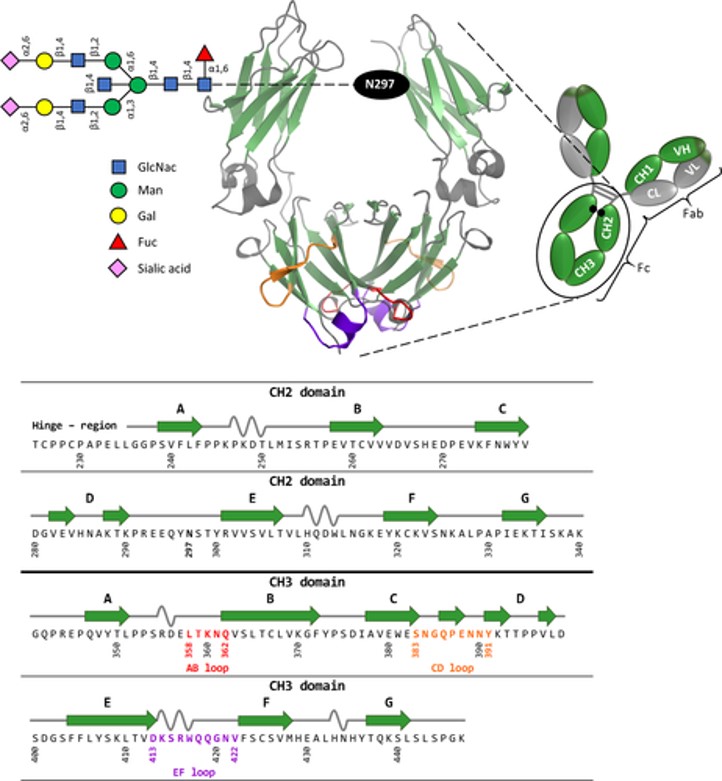
Figure 5: Schematic representation of homodimeric human IgG1-Fc, generated using PyMOL, with the corresponding amino acid sequence [Lobner 2016].
To therapeutically regulate a biological complex in the lab for in vivo translation necessitates a thorough understanding of its structural biology. Clustering of Fcγ receptors occur by binding their ligand – the Fc portion of IgG present in immune complexes or on antibody-coated cells. The activation of FcγR in macrophages result in the secretion of products involved in an inflammatory response to induce phagocytosis and facilitate immune health.
These processes guide the underlying mechanisms of virus, bacteria, and parasite ingestion as well as the antibody-dependent killing of cells that express viral or tumor antigens [Fitzer-Attas 2000]. Phagocytosis leads to antigen processing and presentation to neighboring T cells as well. The Fc gamma Rs also have striking parallels to signaling via T and B cell antigen receptors. The molecules are classified as membranes of the multichain immune recognition receptor family that mediate signaling via the immunoreceptor tyrosine-based activation motif (ITAM) [DeFranco 1995].
Several low affinity receptors such as FcαRI, FcγRIIA, and FcγRIIIA can function as bifunctional receptors to induce activating or inhibiting signals – an adaptable property that can be investigated pre-clinically to attenuate autoimmune and inflammatory diseases [Mkaddem 2014]. The human IgG-Fc immunoglobulins of isotype G is the predominant antibody class in circulation and are compared to two identical light and heavy chains to form a ‘Y-shaped’ structure (Figure 5) [Huber 1976, Lobner 2016]. Through millions of years of evolution, the optimized activity of these very specific and efficient mediators of host protection exerts their influence as a family of cell surface receptors.
In principle, the Fc receptors bind to the Fc portion of antibodies, to function as antigen-antibody immune complexes – providing the humoral immune system with a cellular effector arm to connect the adaptive and innate immune system (Figure 6) [Hogarth 2012]. After binding the immune complexes, the Fc receptors can induce powerful responses to activate, regulate and modulate immunity that often result in the release of cytokines or phagocytosis activation, as well the destruction of antibody-coated targets via antibody-dependent, cell-mediated cytotoxicity to eliminate invading pathogens [Hogarth 2012].
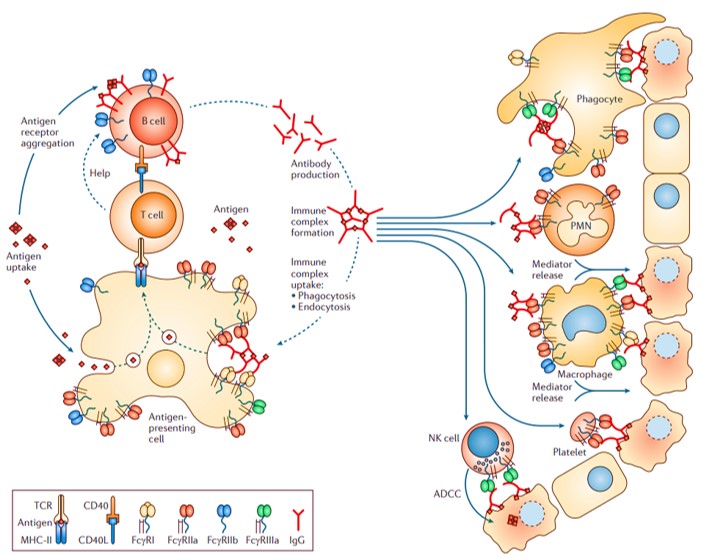
Figure 6: Mechanism-of-action: The role of Fc receptors in normal antibody-based activation of cell responses. [Hogarth 2012].
Fragment crystallizable receptors (FcRs) can be further divided into type I and type II, based on the conformational state of the immunoglobulin Fc domain that interacts with the receptor. Type I fragment crystallized receptors interact with ‘open’ but not ‘closed’ immunoglobulin Fc conformations. Such type I and II human receptors include a range of FcRs from FcγRI-III, FcεRI, FcαRI and FcµR (Figure 7). Furthermore, leukocytes express three principal FcR families; namely FcγR that bind to immunoglobulin G (IgG, and the focus of this post to a large extent), FcεR that binds to immunoglobulin E (IgE), and FcαR that binds to immunoglobulin A (IgA) [Hogarth 2012]. The variable expression of FcγRs in human leukocytes and their properties are classified on table 2.
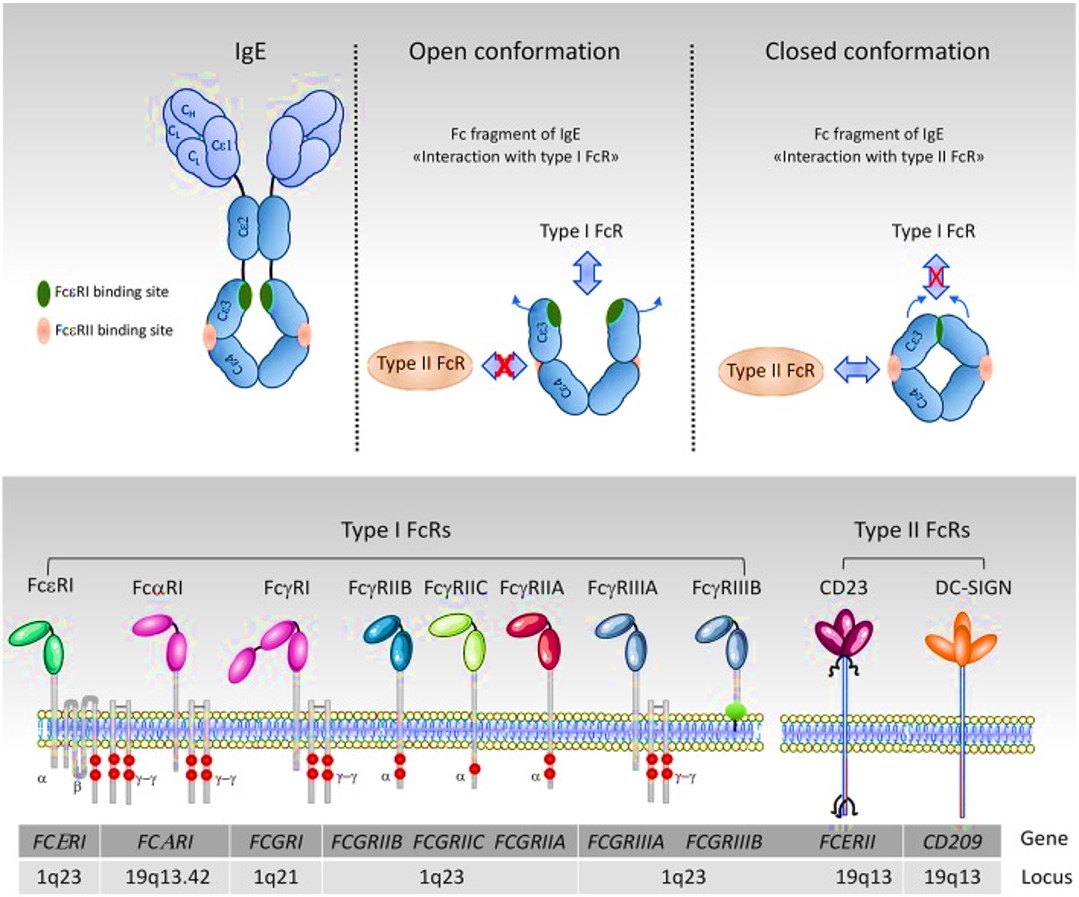
Figure 7: organizational and conformational rearrangements of the IgE Fc. The IgE and the binding sites to FcεRI are in green and to CD23 is in pink. The ‘open’ and ‘closed’ conformations are represented schematically [Mkaddem 2019].
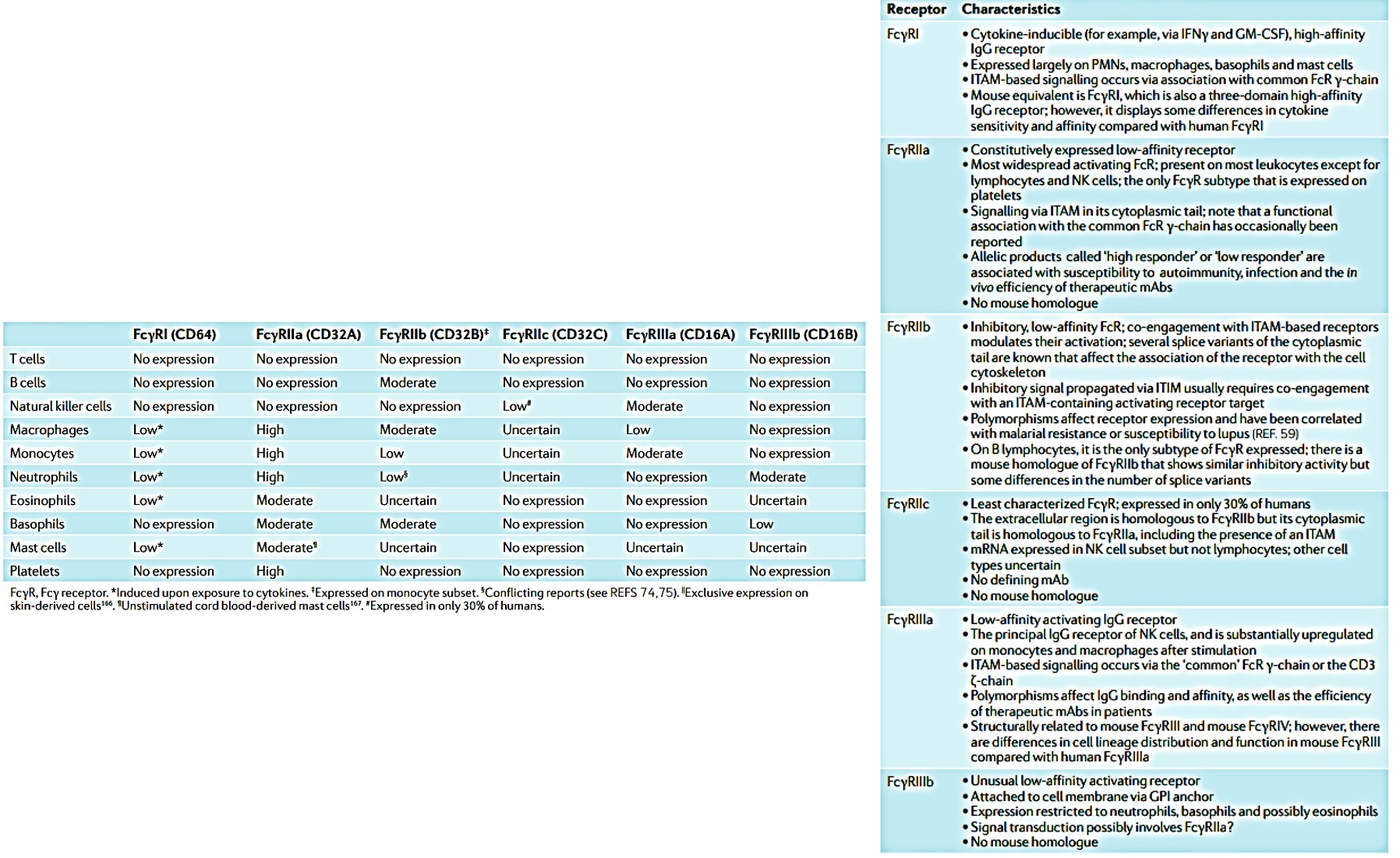
Table 2: (L->R, Zoom in) Distribution and expression of human leukocyte FcγRs and their properties. Key words: CD3, T cell surface glycoprotein CD3; FcγR, Fcγ receptor; GM-CSF, granulocyte–macrophage colony-stimulating factor; GPI, glycosyl phosphatidylinositol; IFNγ, interferon-γ; IgG, immunoglobulin G; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibition motif; mAb, monoclonal antibody; NK, natural killer; PMN, polymorphonuclear neutrophil [Hogarth 2012].
Immune-engineering novel therapies and designing molecular medicines
Immunologists and bioengineers study the dynamics of Fc targeting in the context of drug discovery and immune engineering, relative to three unrelated perspectives. For starters, during autoimmunity or other antibody-dependent hypersensitivity reactions, it is possible to directly block the immunoglobulin-FcR interaction to prevent its activation and prevent an inflammatory response, as seen during the treatment of B-cell lymphoma with monoclonal antibody therapy [Rankin 2006]. Immune engineering can also direct pro-inflammatory responses of cells toward unwanted target cells or pathogens and co-engage the associated activating or inhibitory receptors to direct and fine-tune cellular responses.
The capacity to block the immuoglobulin-FcR interactions have shown promise to regulate major autoimmune inflammatory responses and protect hosts from diseases such as rheumatoid arthritis and systemic lupus erythematosus [Means 2005]. Several therapeutic strategies also intend to directly target pathology with molecular drugs such as small chemical entities, or monoclonal antibodies to block the binding of immune complexes to receptors. The FcR functionalization is important to exert the effects of monoclonal antibodies in the form of immunomodulators. Example drug candidates include monoclonal antibodies with engineered Fcs to selectively bind the activating FcγRs and induce tissue destruction in cancer [Hogarth 2012].
Drug designing strategies can be developed to explore FcR inhibition to counteract the paradoxical effects of its activation during the treatment of inflammation. The development of a new generation of therapies focuses on the classically activated FcRs found on leukocytes to design modules to interfere with or modify the FcγR-IgG interactions to create interesting strategies for drug discovery [Hogarth 2012]. FcRs are characterized as one of the oldest drug targets of the immune system, in support of which pooled human immunoglobulin can be administered intravenously to provide passive protection for immunodeficient patients in the form of intravenous IgG (IVIG). This strategy is now increasingly in use to treat diseases mediated by autoantibodies or immune complexes (Figure 8) [Jolles 2005].
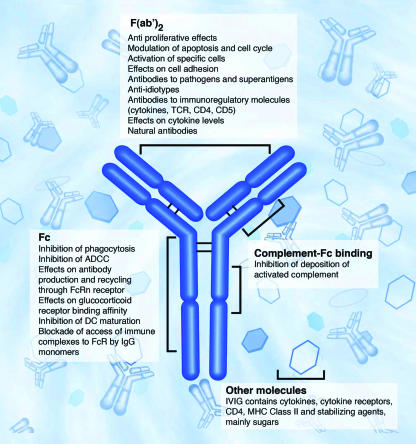
Figure 8: Immunomodulatory actions of intravenous immunoglobulin. Intravenous immunoglobulin (IVIG) may be thought of as four separate components: (1) actions mediated by the variable regions F(ab′)2, (2) actions of Fc region on a range of Fc receptors (FcR), (3) actions mediated by complement binding within the Fc fragment and (4) immunomodulatory substances other than antibody in the IVIG preparations [Jolles 2005].
The Food and Drug administration (FDA) has thus far approved more than 40 monoclonal antibodies (mAbs) since 1986, to treat a variety of diseases, where full-size IgG represents the majority of the approved mAbs. The FDA has, for instance, approved three full-size antigen-binding fragments (Fab) of IgG for clinical use [Lobner 2016], including certolizumab pegol – a tumor necrosis factor blocker to treat patients with moderate-to-severe Crohn’s disease [Melmed 2008], ranibizumab – administered in patients with neovascular age-related macular degeneration [Pieramici 2006], and abciximab – a new antiaggregant agent used in angioplasty [Genetta 1996]. Recent advances in molecular engineering of antibody have also shown the capacity to engineer Fc based antibody domains and fragments as new scaffolds [Ying 2014]. These constructs provide promising therapeutic candidates with enhanced tissue penetration to sterically restrict binding sites for improved therapeutic efficacy.
Molecular engineering scenarios with Fc receptors to block inflammation
The capacity to therapeutically intervene the progression of diseases of the immune system by engineering molecular mechanisms of the underlying FcRs can shed light on the immune-complex mediated functionality. For example, human FcγRIIA transgenic mice have shown hypersensitivity to pathogenic antibodies during the progression of destructive arthritic syndromes. Circulating monocytes from rheumatoid arthritis patients can be introduced to a localized tissue microenvironment on an organ-on-a-chip microfluidic device, or through ex-vivo experimentation to identify FcγRIIA regions responsible to produce reactive oxygen species that upregulate inflammation. Studies have shown how the shift of the FcgammaRIIA-ITAM complex from an activated configuration to an inhibited configuration can circumvent arthritis, a trajectory that can be investigated to develop a range of molecular medicines [Mkaddem 2014].
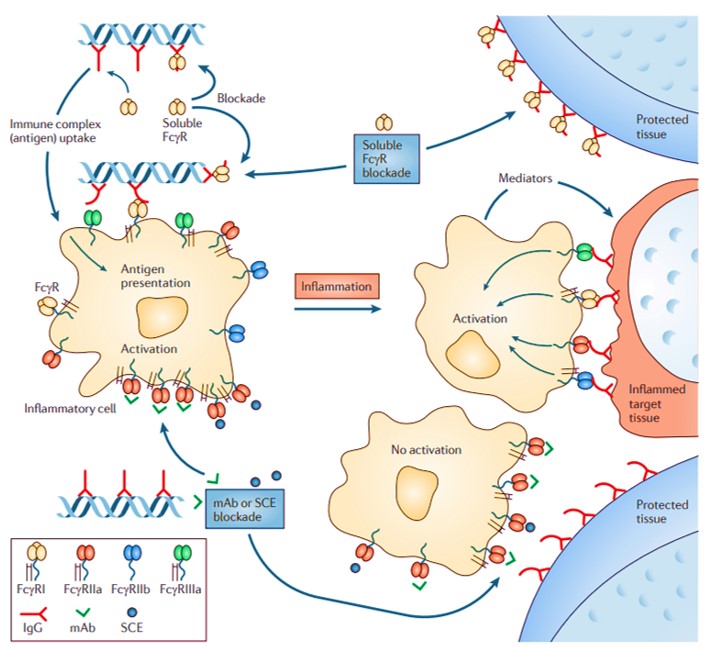
Figure 9: Potential scenarios during the use of soluble Fc receptors to block the immune complex-induced activation of inflammatory cells and tissue destruction. Fc receptors positive antigen-presenting cells can internalize circulating autoantibody-autoantigen immune complexes with anti-chromatin complexes for processing and further antigen presentation. Soluble FcR ectodomain can bind to Fc regions of tissue-bound antibodies to prevent inflammatory cell activation. The cell-bound FcRs can be blocked directly via monoclonal antibodies (mAbs) or small chemical entities that bind to the specific FcR subtypes [Hogarth 2012]
Molecular engineering efforts have also accomplished the development of anti-receptor monoclonal antibodies where intact antibodies, antibody fragments, and small molecules can interact with immunoglobulin-binding domains in activating FcRs. These efforts can block immune-complex mediated cell effects and inflammation, to reverse specific autoimmune disorders such as rheumatoid arthritis [Mkaddem 2014]. Similarly, during allergy treatment, the development of anti-IgE antibodies offer a therapeutic option of recombinant IgE humanized monoclonal antibody E-25, named ‘omalizumab’ across several clinical trials against IgE-mediated allergic reactions [Jardieu 1999]. In its mechanism-of-action, the drug can inhibit IgE binding to FcγRIIA on the surface of mast cells and basophils [Milgrom 1999].
In this instance, the strategy of administering intravenous immunoglobulins (IVIG) resurface as a method to use soluble Fc receptors to block the immune complex-induced activation of inflammatory cells (Figure 9) [Mkaddem 2019]. These IVIGs contain more than 95 percent IgGs and have therapeutic potential to treat a variety of immunomodulatory diseases, including immune thrombocytopenia purpura (ITP), Kawasaki disease and other neurologic diseases [Pecoraro 2017]. In its proposed mechanism-of-action, IVIG can block the activating FcγRs on myeloid cells, to decrease autoantibody-mediated platelet phagocytosis and prevent antibody-dependent cellular cytotoxicity against platelets, for a rapid recovery of platelet count in pediatric patients of ITP [Debre 1993].
In a nutshell, the effects of FcγR in IVIG action highlight the capacity to protect against autoimmune disorders, although some patients can develop immediate or delayed adverse effects to IVIG too [Epstein 2000]. These associated adverse effects depend on the dosage of immunoglobulins and therefore regulating its therapeutic dosage can guarantee efficient therapy to minimize adverse effects. Research works can manipulate the half-life of therapeutic antibodies to enhance patient exposure to their therapeutic effects for longer retention in blood, while administering a cost-effective, reduced dosage [Mkaddem 2019].
Advancing Fc engineering for therapeutic antibody development
The focus on FcRs to prevent pathological responses via immune complexes during autoimmune disease is still in its infancy – this is a surprising observation since most autoimmune-related pathological responses are so readily attributed to this specific receptor type [Hogarth 2012, Chen 2019]. Most studies of soluble FcRs (such as IVIGs) and small molecular entities have nevertheless made their way to the preclinical-to-clinical context, to highlight the role of FcγRs as a potential therapeutic target.
The FcR-mediation in pathological cascades is also highlighted in the transcriptomics data-driven pathological expression of FcγR-mediated phagocytosis in nephrolithiasis [Jeewandara 2023], a deviation from its usual spectrum of disease to highlight the versatility, ubiquity, and importance of studying FcγRs in inflammation-driven diseases. This therapeutic trajectory of FcγR-mediated mechanisms mainly focusses on IgG to underly the clinical utility of many anticancer antibodies, paving the way for a new generation of engineered antibodies that can be fine-tuned for clinical efficacy [Chen 2019]. Monoclonal antibodies are highly effective with minimal side-effects, and Fc Engineering can further enhance their therapeutic potential through antibody-dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), and phagocytosis.
To use antibodies for therapy necessitates the identification of the epitope and the exposition of its mechanism-of-action during infectious diseases [Otsubo 2022]. While the development of antibody therapy began with hybridoma techniques to create cell fusions between murine lymphocytes and myeloma cells. Existing and future efforts of immunotherapy can focus on the broader implications of Fc receptors (FcRs) to develop IgA and IgE antibodies to harness the FcαRI and FcεRI-dependent responses, to induce powerful anti-tumor responses in vitro and in transgenic mouse models with superior outcomes to kill tumor cells through autophagy [Bakema 2011].
Therapeutic IgA also has implications to target gastrointestinal cancers at the mucosal surface [Bakema 2011, MAbs]. Moreover, IgE-FcεRI interactions provide a pharmacologically powerful response in immunity and act as a potent inducer of inflammation, therefore exploring the systems that depend on such interactions (while being mindful of their potential to induce systemic hypersensitivity) are suited for further investigation [Hogarth 2012]. Newer Fc engineering protocols have emerged for antibody skeleton engineering and Fc glycoengineering that rely on amino acid substitutions at the Fcγ receptor binding site within the Fc domain of the antibody and also focus on regulating the glycan composition to optimize antibody-based therapeutics, respectively. The constructs result in optimized characteristics for specific requirements, as outlined (Figure 10).

Figure 10: A representation of advanced Fc Engineering methods [Creative Biolabs].
Mechanistically, an interesting property of antibodies and the FcR system is the ‘scorpion effect,’ where an antibody recognizes a cell surface molecule via its antigen-binding sites to simultaneously engage an Fc receptor on the same cell and create a heterotrimeric complex; a phenomenon that can be therapeutically investigated. The ‘scorpion-like’ arrangement activated the cell by recruiting an activating Fc receptor and has greater implications in the context of antibody engineering [Kurlander 1983]. These compounds and their conformations can be engineered to selectively engage activating or inhibitory Fc receptors to provide an enhanced therapeutic effect for the improved inhibition of inflammation or for more effective target cell apoptosis (Figure 11) [Hogarth 2012].
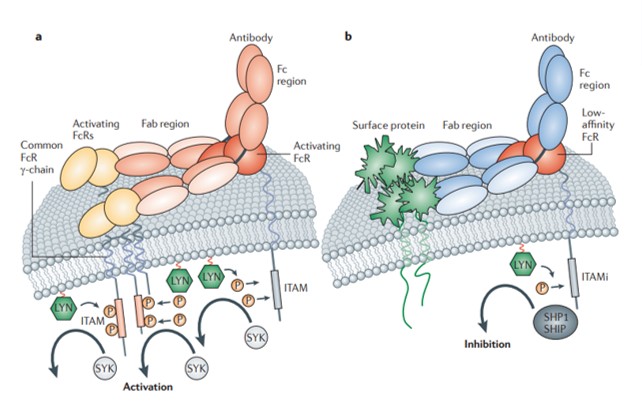
Figure 11: The ‘scorpion effect’ where an anti-receptor antibody engages its target via antigen-binding sites to form a trimolecular complex. Where a) the formation results in cell activation or b) result in inhibitory function for therapeutic impact [Hogarth 2012].
Wrapping it up with combined bioengineering and microfluidics-assisted antibody discovery
Since antibody-derived biologics are soon becoming a prominent class in molecular medicine in the fight against cancer and autoimmune diseases [Josephides 2020], with monoclonal antibodies outlined as a therapeutic option in various diseases for the targeted treatment with minimal side-effects, as a means of precision medicine. Bioengineers are keen to develop efficient antibody discovery and cell-line development pipelines to successfully translate antibodies into immunotherapies from the bench-to-bedside.
A significant challenge in the workflow is to screen large cell populations for productivity, antigen specificity, and then isolate rare cells with ensured monoclonality [Evans 2015]. The innovative capacity to introduce an integrated pico-droplet system for high throughput single-cell analysis, coupled with cell sorting, dispensing and monoclonal assurance can therefore overcome several existing bottlenecks of the workflow, as afforded via the microfluidics integrated CytoMine instrument – a single cell analysis and assurance system, within a single compact system (Figure 12) [Josephides 2020].
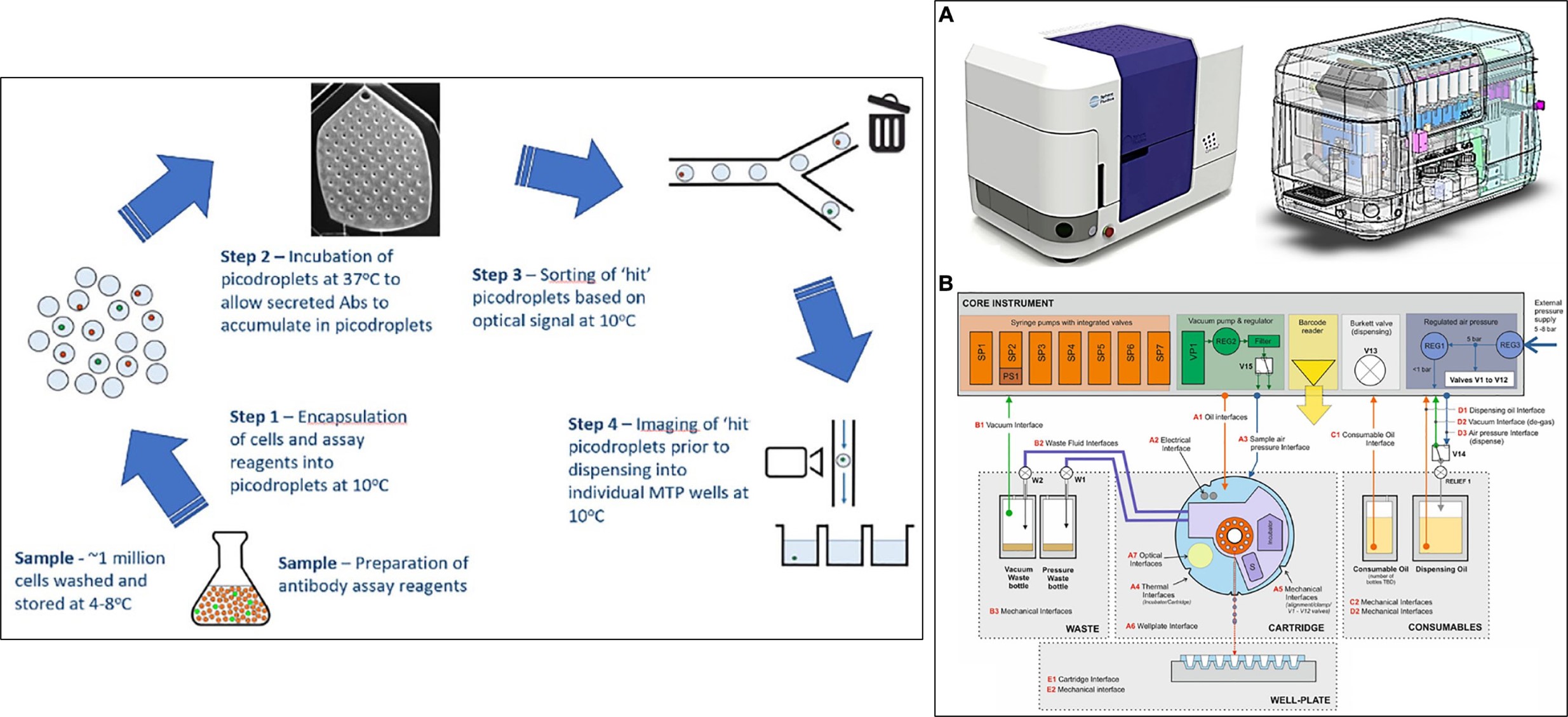
Figure 12: The CytoMine workflow integrates encapsulation, screening, sorting, isolation, and verification of high-secreting clones of cells in a fully automated process. The entire workflow is carried out at -100C, except the incubation step at 370C to ensure that the cells do not divide and are not actively secreting through various stages of the process. A) the CytoMine instrument, B) A schematic representation of the core of the CytoMine instrument [Josephides 2020].
In its mechanism-of-action, the instrument seamlessly encapsulates cell culture samples originally prepared in a preferred culture medium into pico-droplets of culture medium by pumping them through microfluidic channels [Josephides 2020]. A large cohort of cells (hundreds of thousands to several millions) can be encapsulated and processed in a single run. These pico-droplets can be incubated to facilitate the cellular secretion of antibody molecules. The small volume of the pico-droplets allows antibodies produced by a single cell to reach a detectable concentration within a short timeframe.
This method includes cell sorting via Förster resonance energy transfer (FRET) reagents to sort the positive cells and select the desired pico-droplets that are actively channeled for collection. Cells that produce antibodies and secrete antibody molecules into the pico-droplets can be detected via the FRET signals. For example, the IgG production of single cells can be measured in pico-droplets via a customized pair of IgG-specific fluorescent probes [Figure 13].
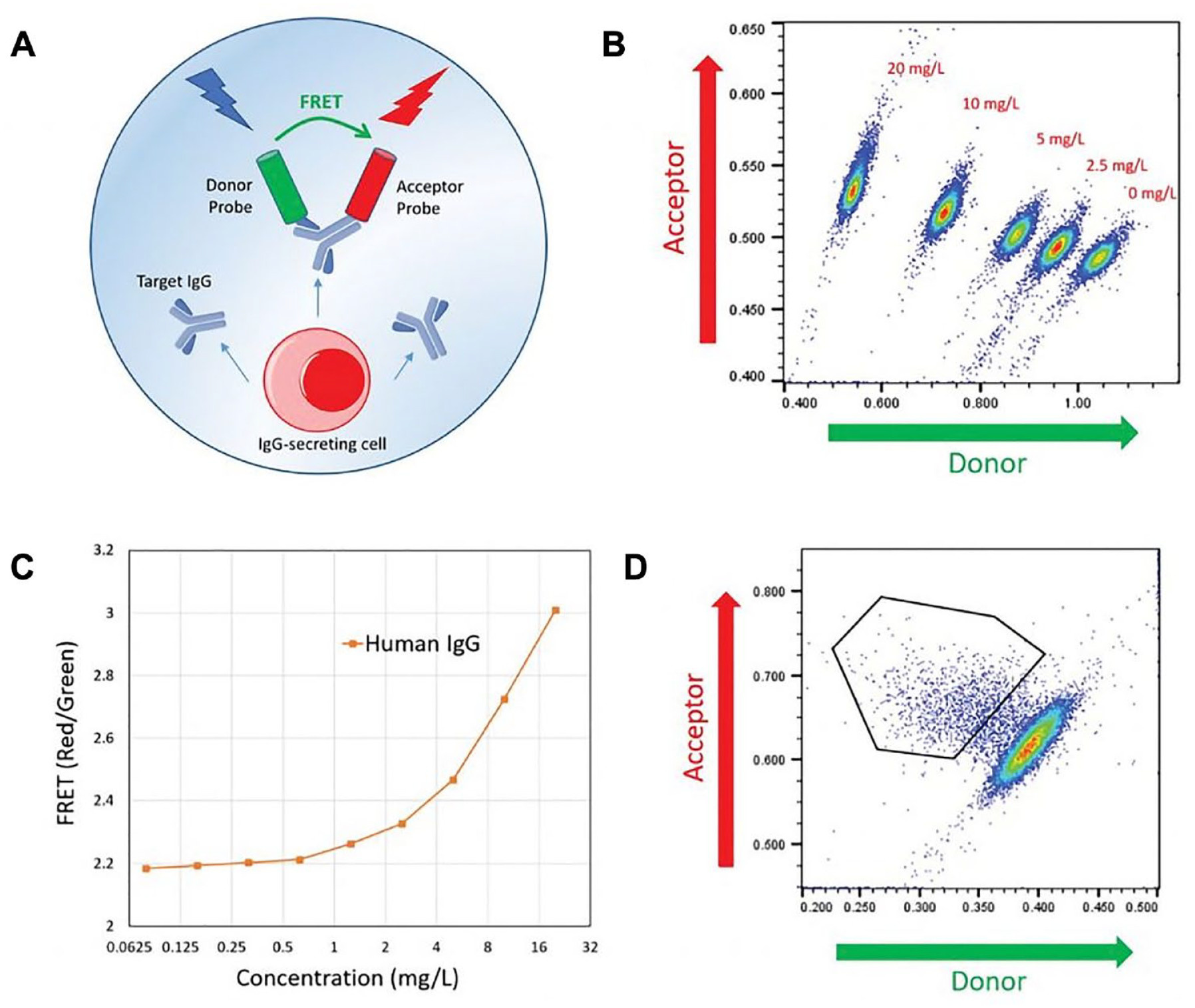
Figure 13: An example cyto-mine IgG secretion assay A) human IgG-specific probes within each pico-droplet bind to secreted IgG to form a three-body FRET complex. B) Scatterplots of FRET signal for pico-droplets contain the indicated concentrations of human IgG with IgG detection probes. C) Standard titration curve D) Scatterplot generated from pico-droplet-encapsulated Chinese Hamster Ovary cells incubated with IgG-specific detection probes [Josephides 2020].
Although the preparation of monoclonal antibodies mainly depends on hybridoma methods that preserve the biological activity and increase the antibody yield as a powerful, mature development pipeline. The methods are time-consuming with low-throughput and limit the antibody screening process. Recent works have therefore sought higher efficiency by integrating microfluidics-assisted instruments such as CytoMine [Josephides 2020, Gaa 2021] and DropMap [Eyer 2017] for rapid antibody discovery and immune monitoring via single-cell phenotyping. Added to that, the recent introduction of a wholly new bioengineered microfluidic chip for monoclonal antibody functional screening can combine hybridoma technology with single-cell technology as a micro-array-based approach to improve the screening throughput, with reduced screening time [Xi 2024].
In this way, biomedical engineering can further accelerate efforts to advance immunotherapy with streamlined technologies. Microfluidics approaches offer high accuracy via single cell compartmentalization, to obtain deeper insights across diverse species [Eyer 2017]. The versatile instruments can maintain broad datasets at the intersection of immunology and biomedical engineering, to establish the methods as standard practice for rapid antibody discovery and to carryout Fc receptor engineering for precision healthcare.
Header Image: The future of antibody engineering today: Designing custom antibody tools for life science applications - with a downloadable booklet available via Science Magazine.
References
- Guyre C. et al. FcγR-directed immunotherapies, The Immunoglobulin Receptors and their Physiological and Pathological Roles in Immunity, SpringerLink, 1998
- Fitzer-Attas C. et al. Fcγ Receptor–Mediated Phagocytosis in Macrophages Lacking the Src Family Tyrosine Kinases Hck, Fgr, and Lyn, Journal of Experimental Medicine, 2000.
- Sips M. et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies, Mucosal Immunology, 2016
- Jeewandara T. et al. The dynamics of pathological biomineralization in the renal papillae, World Congress of Nephrology 2023, abstract number: WCN23:0323, 2023.
- Khan S. et al. Randall's plaque and calcium oxalate stone formation: role for immunity and inflammation, Nature Reviews Nephrology, 2021.
- Nimmerjahn F. et al. Fcgamma receptors as regulators of immune responses, Nature Revies Immunology, 2008
- Jeewandara T.M., Immune Engineering - Development of Immuno-Materials, Nature Research Communities, SpringerLink, Access: https://communities.springernature.com/posts/immune-engineering-development-of-immuno-materials 2017
- Harrington R. et al. Targeting Inflammation in Coronary Artery Disease, NEJM, 2017
- He Y. et al. Myeloid Piezo1 Deletion Protects Renal Fibrosis by Restraining Macrophage Infiltration and Activation, Hypertension, 2022.
- Vogelpoel L. et al. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages, Nature Communications, 2014
- Khan S. et al. Reactive oxygen species, inflammation, and calcium oxalate nephrolithiasis Translational Andrology and Urology, 2014
- Mkaddem S. et al. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools, Frontiers in Immunology 2019
- Hoepl W. et al. FcγR-TLR Cross-Talk Enhances TNF Production by Human Monocyte-Derived DCs via IRF5-Dependent Gene Transcription and Glycolytic Reprogramming, Frontiers in Immunology, 2019
- McInnes I et al. The pathogenesis of rheumatoid arthritis, New England Journal of Medicine, 2011
- Weinblatt M. et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis New England Journal of Medicine, 2010
- Lobner E. et al. Engineered IgG1-Fc – one fragment to bind them all, Immunological Reviews, 2016
- DeFranco A. Transmembrane signaling by antigen receptors of B and T lymphocytes, Current Opinion in Cell Biology, 1995
- Huber R. et al. Crystallographic structure studies of an IgG molecule and an Fc fragment, Nature, 1976
- Hogarth M. et al. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond, Nature Reviews Drug Discovery, 2012
- Rankin C. et al. CD32B, the human inhibitory Fc-gamma receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma, Blood, 2006
- Means T. et al. Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9, The Journal of Clinical Investigation 2005
- Jolles S. et al. Clinical uses of intravenous immunoglobulin, Clinical and Experimental Immunology, 2005
- Melmed G. et al. Certolizumab pegol, Nature Reviews Drug Discovery, 2008
- Pieramici D. et al. Ranibizumab: treatment in patients with neovascular age-related macular degeneration, Expert Opinion on Biological Therapy, 2006
- Genetta T. et al. ABCIXIMAB: a new antiaggregant used in angioplasty, The Annals of Pharmacotherapy,1996
- Ying T. et al. Engineered Fc based antibody domains and fragments as novel scaffolds, Biochimica et Biophysica Acta (BBA), 2014
- Mkaddem S. et al. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools, Frontiers in Immunology, 2014
- Jardieu P. et al. IgE inhibition as a therapy for allergic disease, International Journal of Allergy, and Immunology, 1999
- Milgrom H. et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group, New England Journal of Medicine, 1999
- Pecoraro A. et al. Immunoglobulin replacement therapy in primary and secondary antibody deficiency: The correct clinical approach, International Immunopharmacology, 2017
- Debre M. et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura, Lancet, 1993
- Epstein J. et al. Important drug warning: Immune Globulin Intravenous (human) (IGIV) products, Neonatal networks, 2000
- Chen X. et al. FcγR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy, Frontiers in Immunology, 2019
- Otsubo R. et al. Monoclonal antibody therapeutics for infectious diseases: Beyond normal human immunoglobulin, Pharmacological Therapy, 2022.
- Bakema J. et al. Targeting FcαRI on polymorphonuclear cells induces tumor cell killing through autophagy, The Journal of Immunology, 2011
- Bakema J. et al. Immunoglobulin A: A next generation of therapeutic antibodies? MAbs, 2011.
- Kurlander R. et al. Blockade of Fc receptor-mediated binding to U-937 cells by murine monoclonal antibodies directed against a variety of surface antigens, Journal of Immunology, 1983
- Josephides D. et al. Cyto-Mine: an integrated, picodroplet system for high-throughput single-cell analysis, sorting, dispensing, and monoclonality assurance, SLAS TECHNOLOGY: Translating Life Sciences Innovation, 2020
- Evans K. et al. Assurance of monoclonality in one round of cloning through cell sorting for single cell deposition coupled with high resolution cell imaging, Biotechnology Progress, 2015
- Gaa R. et al. Versatile and rapid microfluidics-assisted antibody discovery, MAbs 2021
- Eyer K. et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring, Nature Biotechnology, 2017
- Xi J. et al. A Microfluidic chip for monoclonal antibody functional screening, ICBBB '24: Proceedings of the 2024 14th International Conference on Bioscience, Biochemistry and Bioinformatics, 2024
Follow the Topic
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcement
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in