Implantable biomedical system to regain tactile sensing for paralyzed people
Published in Bioengineering & Biotechnology

Paralysis is a substantial health problem all over the world. In the United States alone, approximately 5.4 million people live with paralysis – nearly 1 in 50 people. To restore daily activities for paralyzed people both controlled stimulation of muscle to induce movement and somatosensory feedback to the brain of hand-object interaction are required. Utilizing brain machine interface (BMI)-controlled stimulation, paralyzed individuals can regain hand movement with their native hand without sensation 1-3. However, sensory feedback into these BMI-controlled stimulators remains an unmet challenge. While wearable sensors have emerged as potential solutions for delivering tactile functionality noninvasively, they come with certain disadvantages, including limited longevity, uncomfortable materials, and the added bulk of sensorized gloves.
We team’s approach deviates from current trends: we aim to develop the first implantable sensor system to restore touch to reanimated hands. Signals captured by the sensors would be wirelessly transmitted to a brain stimulator that activates a somatosensory region in correlation to the sensor output, resulting in a sense of touch (Figure 1a). This ambitious goal requires interdisciplinary collaboration to integrate an implantable micro-electro-mechanical-systems (MEMS) sensor for movement sensing and neuron stimulation, ultra-low power wireless integrated circuits for signal and power communication and in-depth study of the physiology involved in neuron and muscle behavior. Since 2017, we've been lucky to foster a partnership between experts like Dr. Lucas and Dr. Richardson from Penn Medical School and Dr. Allen and Dr. Aflatouni group from Penn Engineering School. Our current focus is to implement the in-situ functionality of our implantable pressure sensing system, ensuring accurate force transduction and seamless wireless operation, while also initiating preliminary in vivo testing.
Our sensing system comprises three distinct components (Figure 1b-c): a parallel plate capacitive force sensor contains an upper silica plate and a middle silica plate with a cavity in between; a miniaturized, wireless transfer module including an application-specific integrated circuit for power and data communication between the implantable sensor and wearable base unit4; and a three-layer package fully encapsulated with biocompatible and hermetic silica material based on laser-assisted fusion bonding technology5.
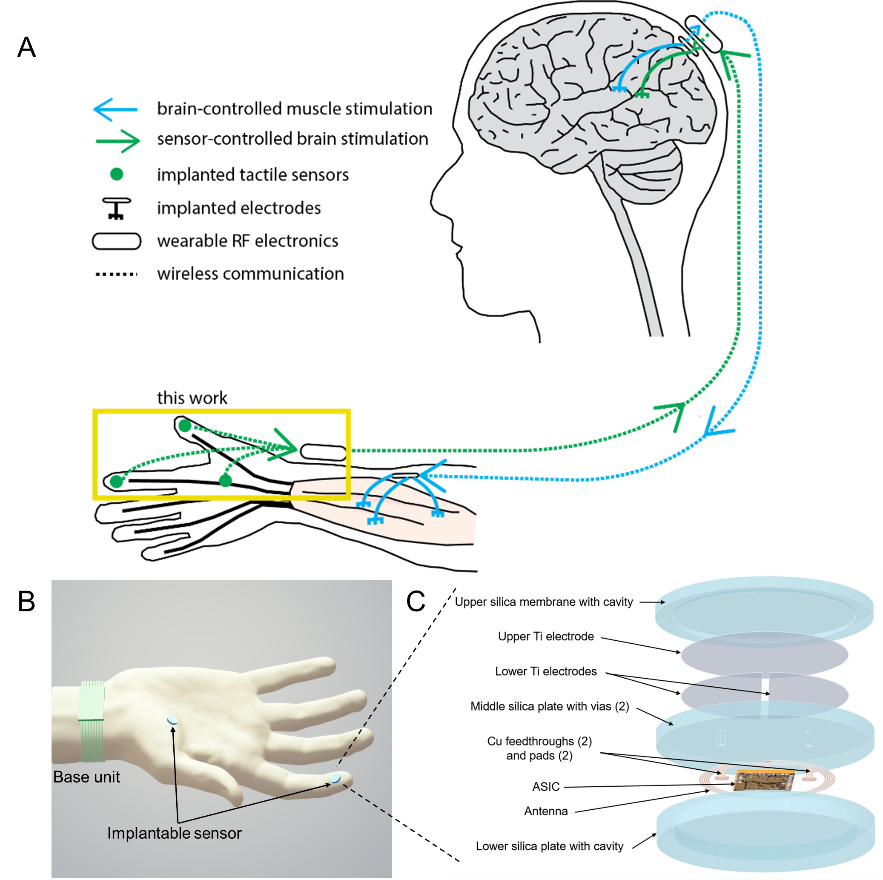 Figure 1. Implantable, wireless, battery-free tactile sensing system. a Illustration of envisioned closed-loop hand reanimation treatment for paralysis. The implantable wireless tactile sensors developed in this work are intended to be part of an artificial sensory feedback pathway (green). The tactile signals acquired by the sensors would be wirelessly conveyed to a brain stimulator that activates a somatosensory area in proportion to the sensor output, resulting in a sense of touch. In addition, volitional movement would be restored by stimulating paralyzed muscle in proportion to neural activity recorded in a motor brain area (blue). b Illustration of the tactile sensing system wirelessly monitoring normal forces acting on the fingertip and palm. The implantable sensing system integrates capacitive force detection, signal processing, and customized wireless data and power transceiver interfaced to a wearable base unit. c Exploded view of the implantable sensor, which contains five layers: an upper silica membrane, capacitive double plates, a middle silica plate (with vias, feedthroughs, and pads), an ASIC with antenna and electronic components, and a lower silica plate.
Figure 1. Implantable, wireless, battery-free tactile sensing system. a Illustration of envisioned closed-loop hand reanimation treatment for paralysis. The implantable wireless tactile sensors developed in this work are intended to be part of an artificial sensory feedback pathway (green). The tactile signals acquired by the sensors would be wirelessly conveyed to a brain stimulator that activates a somatosensory area in proportion to the sensor output, resulting in a sense of touch. In addition, volitional movement would be restored by stimulating paralyzed muscle in proportion to neural activity recorded in a motor brain area (blue). b Illustration of the tactile sensing system wirelessly monitoring normal forces acting on the fingertip and palm. The implantable sensing system integrates capacitive force detection, signal processing, and customized wireless data and power transceiver interfaced to a wearable base unit. c Exploded view of the implantable sensor, which contains five layers: an upper silica membrane, capacitive double plates, a middle silica plate (with vias, feedthroughs, and pads), an ASIC with antenna and electronic components, and a lower silica plate.
To benefit more paralyzed people in the real world, we search for appropriate implantable materials that have already been applied in the FDA approved implantable medical systems. To satisfy the long-term hermicity and biocompatibility requirements, materials such as titanium, alumina, and fused silica are the most popular materials. To enable the wireless transmission, we selected fused silica as the package material, which is transparent at both radio frequency and optical frequency with superior thermal and mechanical properties. While the fabrication of the material is difficult, in this technology, we introduce laser micromachining in addition to the traditional MEMS fabrication process.
Typically, inside implantable MEMS systems, there is an implantable sensor or stimulator for movement detection or neuron stimulation, an energy source (either a battery or a wireless power transmission system) for powering the electronics as well as wireless signal communication electronics. One of the primary challenges was achieving the system hermetic seal with silica without compromising the electronic circuitry’s functionality. Traditional hermetic sealing with silica relies on fusion bonding, requiring temperatures above 1000 °C which is incompatible with CMOS devices. To address this issue, we propose a localized fusion bonding method using lasers. This approach delivers high temperature locally to encapsulate the electronics devices, keeping them intact from the fabrication process. For a design that aligns with fingertip implantation using silica, we opted for a parallel plate capacitive pressure sensor. While conventional designs have electrodes on both the top and bottom plates of the sensor, this configuration necessitates a feedthrough structure on the sensing membrane which may impact the sensitivity and increase fabrication complexity. To solve this problem, our design incorporates a floating potential electrode for the top plate and dual electrodes for the bottom plate. This ensures a smooth sensing membrane, eliminating the need for feedthrough fabrication and guaranteeing stable and consistent performance.
With all these issues addressed, we successfully developed an implantable sensing system, with all its components housed in a silica package of 6 mm in diameter and 1.7 mm in thickness. Following its completion, we conducted in vivo experiments to assess its performance initiated with rats. Given that a rat's palm isn't sufficiently large to accommodate the sensor, we implanted the system in its hindlimb. During the evaluation, we used a neonatal-sized blood pressure cuff to exert controlled pressure. However, the consistency of these results was poor. The sensor frequently shifted upon pressure application, primarily because rat skin is thin and more loosely connected to the underlying muscle and bone by connective tissue. We concluded that rodents are not an ideal model for testing this tactile sensor. A primate's hand, being the intended target, presents a more suitable model for thorough assessment. The sensor was implanted into the fingertip of a fresh (not formalin-fixed) macaque monkey hand (Figure 2a). Time-varying forces were applied with a load cell recording applied forces. The sensor capacitance changes measured wirelessly by an external base unit closely followed the dynamic forces (Figure 2b).
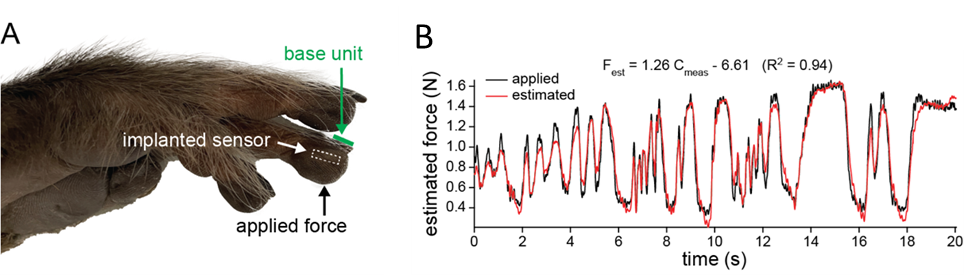
Figure 2. Ex vivo sensor performance. a Image of cadaver monkey hand after the wireless sensor was surgically placed in the indicated fingertip. The base unit was placed on the fingernail and static and dynamic forces were applied to the skin overlying the implanted sensor. b The sensor response to dynamic forces. Estimated force based on the sensor output compared to the applied force.
The results highlight the system’s repeatability and consistency. The sensor is intended for use in advanced hand reanimation treatment for paralysis. The system we’ve developed represents just one part of this comprehensive treatment. Our ongoing mission is to enhance the daily lives of those with paralysis.
References
1 Hochberg L. R. et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372-375 (2012).
2 Bouton C. E. et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247-250 (2016).
3 Ajiboye, B. et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet 389, 1821–1830 (2017).
4 Hao, H. et al. A wireless artificial mechanoreceptor in 180-nm CMOS. IEEE Trans. Microw. Theory Techniques 69, 2907–2920 (2021).
5 Du, L. & Allen, M. G. CMOS compatible hermetic packages based on localized fusion bonding of fused silica. J. Microelectromech. Syst. 28, 656–665 (2019).
Follow the Topic
-
Microsystems & Nanoengineering

This journal, with a target for a high-end journal for years to come, seeks to promote research on all aspects of microsystems and nanoengineering from fundamental to applied research.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in