Improving the treatment of prosthetic joint infection
Published in Bioengineering & Biotechnology

I was involved in the care of a patient with periprosthetic joint infection during the second year of medical school. Despite the excellent level of care, the patient felt unsatisfied owing to the prolonged hospital stay for multiple revision surgeries that were part of the two-stage revision procedures1. Around the same time, I was preparing to start my PhD work and was discussing the most suitable PhD thesis project with my principal investigators, Drs. Ebru Oral and Orhun Muratoglu at the Harris Orthopedics Laboratory. Both my principal investigators are world-leading experts of prosthetic joints, particularly ultra-high-molecular-weight-based prosthetic joints2-4. We wanted to explore the possibilities of creating a high-strength, high-wear, drug-eluting material based on ultra-high-molecular-weight polyethylene. One of the main translational applications of this material is to create antibiotic-eluting prosthetic joints for the treatment of periprosthetic joint infection.
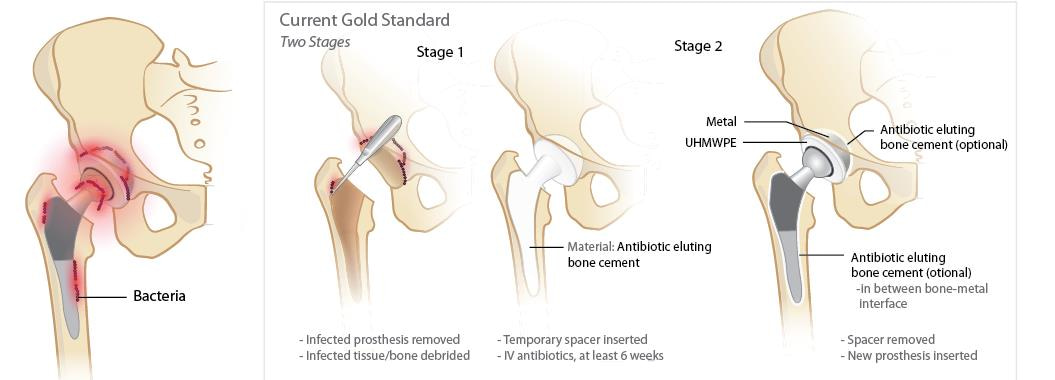
Figure 1. Schematic of the current gold-standard treatment of patients with periprosthetic joint infection. When the clinical diagnosis of prosthetic joint infection is confirmed, orthopedic surgeons remove the infected joint prosthesis, debride the necrotic and infected tissues, and implant an antibiotic-eluting bone cement. Patients also get a combined intravenous (IV) and oral antibiotics for at least six weeks. After bacterial eradication is confirmed, the orthopedic surgeon would re-open the joint site, remove the antibiotic-eluting bone cement, and implant a new permanent prosthetic joint. Patients would then get a combination of IV and oral antibiotics to prevent re-infection of the new prosthetic joint5.
We spent close to a year going through iterations of modifiable parameters to get a material that maximizes the percent accessible drugs while minimizing the sacrifice in mechanical strength and wear resistance. After fabricating several promising candidates for further testing, we met routinely with a team of orthopedic surgeons at the Massachusetts General Hospital (MGH); Andrew Freiberg, Henrik Malchau and Harry Rubash, to design and execute what we felt was the most clinically relevant testing. As we designed the experiments, we realized that we needed microscopy and imaging experts to assist us in imaging the bacterial biofilm on explants and to aid us in utilizing the best methods for imaging live bacteria. This is where I decided to reach out for help from my medical school and PhD classmate, Sheldon Kwok, and his principal investigator, Andy Yun. Sheldon was working with Prof. Andy Yun on several projects that also involved two-photon fluorescence microscopy. I am truly fortunate that both of them agreed to help me perform imaging of implant-adherent bacteria, and determine its viability status using live–dead stains.
When we finished the in vitro experiments and decided to move into in vivo studies, I realized that I needed major help from people experienced in animal studies and orthopedic surgery. Despite my ultimate professional goal of becoming an academic orthopedic surgeon, I am still in the very early stages of my medical training, and was not comfortable performing animal surgery on my own. This is where an experienced member of the Harris Orthopedics Lab, David Bichara, came to the rescue. David has had at least a decade of animal surgery experience, and fortunately, he agreed to help me perform the animal surgeries.
In the end, as we all sat down together and reviewed all the data, we were very happy to have achieved what we aimed for: a fully functional antibiotic-eluting prosthetic joint. Our last iteration of this material showed only marginally superior drug elution when compared to antibiotic-eluting bone cement, but the mechanical strength was several folds higher. In fact, the mechanical strength of our reported material is within the mechanical strength of clinically proven, permanent ultra-high molecular weight polyethylene (UHMWPE) prosthetic joints. I hope that this material can fulfill its promise to patients like the one that inspired me to work on peri-prosthetic infection, by at least providing comfort and mobility during the treatment of this very difficult condition.
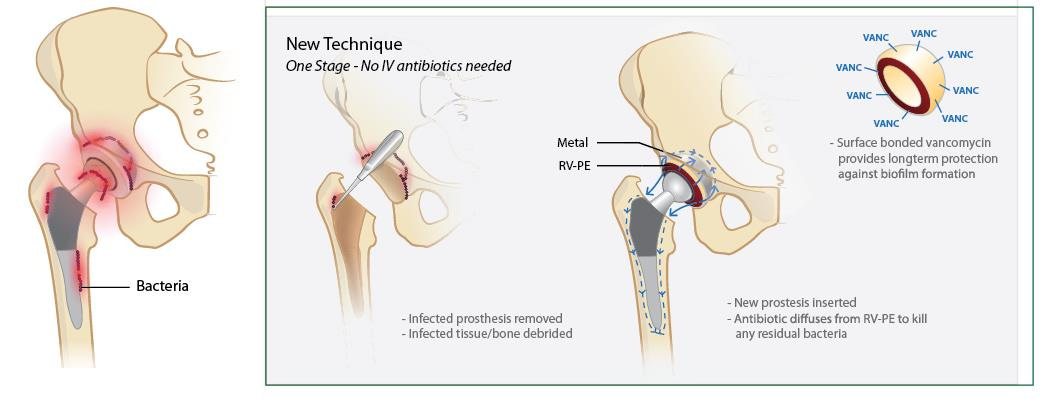
Figure 2. Schematic of our proposed treatment of patients with periprosthetic joint infection using our new material. When clinical diagnosis of prosthetic joint infection is confirmed, orthopedic surgeons would remove the infected joint prosthesis, debride the necrotic and infected tissues, and implant the antibiotic-eluting prosthetic joints. No antibiotic-eluting fixation is needed, unlike most one-stage replacement strategies currently used. Furthermore, the surface-grafted antibiotics provided extended prevention of bacterial recolonization of the prosthetic joint.
I have had the fortune of being surrounded by very talented and smart people. This project would have never come to fruition without the help of all the authors. I hope that our findings and new material will be the initial stepping-stone toward a human use-approved, antibiotic-eluting prosthetic joint.
References
- Cooper, H.J., Della Valle, C.J. The two-stage standard in revision total hip replacement. Bone Joint J. 95-B, 84-87 (2013).
- Oral, E., et al. α-Tocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials. 25, 5515-5522 (2004)
- Oral, E., et al. Diffusion of vitamin E in ultra-high molecular weight polyethylene. Biomaterials. 27, 5225-5237 (2007)
- Muratoglu O.K., et al. A novel method of cross-linking ultra-high molecular weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty. 16, 149-160 (2001).
- Osmon D.R., et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases society of America. Clinical Infectious Disease. 56, e1-25 (2013).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in