In Situ Partial‑Cyclized Polymerized Acrylonitrile‑Coated NCM811 Cathode for High‑Temperature≥100 °C Stable Solid‑State Lithium Metal Batteries
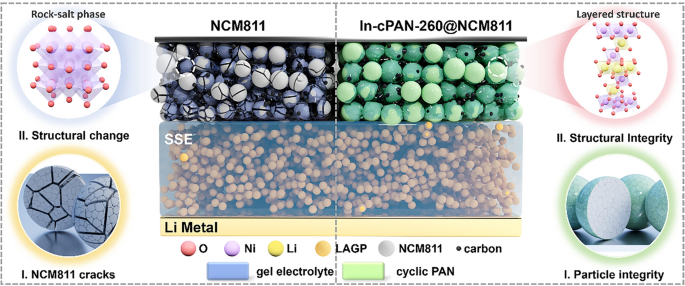
As electric vehicles, aerospace and underground instruments demand energy-dense power that survives extreme heat, the cathode–electrolyte interface in solid-state lithium metal batteries (SSLMBs) becomes the weakest link. Now, a National University of Defense Technology team led by Prof. Qingpeng Guo and Prof. Chunman Zheng unveils an ultrathin, ion-electron dual-conductive skin that keeps Ni-rich NCM811 stable even at 100 °C. Their work, published in Nano-Micro Letters, offers a scalable polymerization-cyclization recipe ready for roll-to-roll production.
Why the New Coating Matters
• High-Temperature Resilience: In-cPAN-260@NCM811 retains 86.8 % capacity after 300 cycles at 60 °C and 75 % after 100 cycles at 100 °C—far above the failure point of conventional oxide cathodes.
• Dendrite & Cracking Shield: A 12–15 nm cPAN network coordinates with surface Ni, raising the Ni-migration barrier from 2.83 eV to 3.36 eV and suppressing intergranular fracture under 4.3 V.
• Mixed Conduction: Partially graphitized cPAN delivers 0.14 S cm-1 electronic and 1.22 × 10-4 S cm-1 ionic conductivity, cutting charge-transfer resistance by 40 %.
Innovative Design & Features
• In-situ Polymerization: Acrylonitrile polymerizes directly on NCM811 in LiFSI-containing solvent, ensuring conformal coverage without extra binders.
• Controlled Cyclization: 260 °C air treatment converts 80 % of –CN into C=N/C=C conjugated rings, proven by FTIR, Raman and N 1s XPS.
• PVDF-HFP/LAGP Composite Electrolyte: The 50 µm membrane provides 0.78 mS cm-1 at 60 °C and stable Li plating for 3000 h.
Applications & Safety Validation
• Pouch-Cell Abuse Tests: LED arrays stay lit after folding, cutting and nail penetration; volume expansion <1 % versus 570 % for liquid cells during 90 °C storage.
• ARC Calorimetry: Self-heating onset rises from 160.9 °C (liquid) to 255.6 °C (solid), with peak runaway temperature dropping 60 %.
• Rate Capability: 163 mAh g-1 at 0.1 C, 140 mAh g-1 at 1 C, and only 15.7 mV polarization shift after 50 cycles.
Challenges & Outlook
The team highlights the need for thinner coatings (<5 nm) to push energy density above 400 Wh kg-1 and for continuous coating lines compatible with dry-electrode calendaring. Future research will couple real-time impedance monitoring with AI-driven cyclization control to extend cycle life beyond 1 000 at 120 °C.
This facile surface-engineering strategy opens a commercially viable route for thermally robust, high-energy SSLMBs in extreme-environment applications.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in