Insights into the protective immune response by immunization with full-length recombinant TprK protein: cellular and humoral responses
Published in Immunology
Syphilis is a systemic sexually transmitted disease, caused by the spirochere Treponema pallidum subsp. pallidum (T. pallidum)1. Due to the difficulty in long-term cultivation in vitro with a cell-free system, many conventional tools are not available for the exploration of the pathogenesis of T. pallidum, which also greatly hindered the development of the syphilis vaccine2,3.
With the development of structural modeling of proteins, more information about putative surface-exposed proteins as candidate vaccinogens is being obtained4. Among them, TprK may be a controversial protein not only for the location of the proteins but also for its role in the syphilis vaccine. Two laboratories both used the recombined TprK to immunize the animals, however, the results were the exact opposite5,6. The reason behind this has yet to be perfectly explained. Despite this, current experimental technology has become more varied and more mature. The protective immune response induced by immunization with TprK is worth careful and comprehensive exploration.
Because the tprK gene is highly heterogenous intra-strain, our team members discussed and decided to use the full-length tprK gene of the standard Nichols strain, which was revealed to have almost a single tprK sequence, as a template for the expression of recombinant TprK protein (rTprK) first7,8. And the rabbit-infected models are well-acknowledged for the syphilis research9. By employing immunized-infected rabbit models, we sought to fully investigate the role of TprK in the protective immune response against T. pallidum and revealed the induced cellular and humoral immune response, thus providing important information for understanding the immunogenicity of TprK.
After confirming the availability of the purified rTprK, we began to immunize the rabbit until the titer of the anti-TprK antibody reached 1:200,000. It took approximately six weeks. Then 10 days after the final immunization (on February 18th, 2022), all rTprK-immunized rabbits and control rabbits were anaesthetized with acepromazine via intramuscular injection at 1 to 3 mg/kg body weight and challenged intradermally with 0.1 mL of 1×107 /mL fresh T. pallidum (Nichols strain) at 10 sites on their shaved backs. The following observations and measures were according to Figure 1 shown. This animal experiment took more than six months. All team members worked hard to arrange the experiment plans based on the obtained results, especially Prof. Yang and Dr. Tong, who not only proposed the idea but provided many good suggestions for improving experimental protocols.
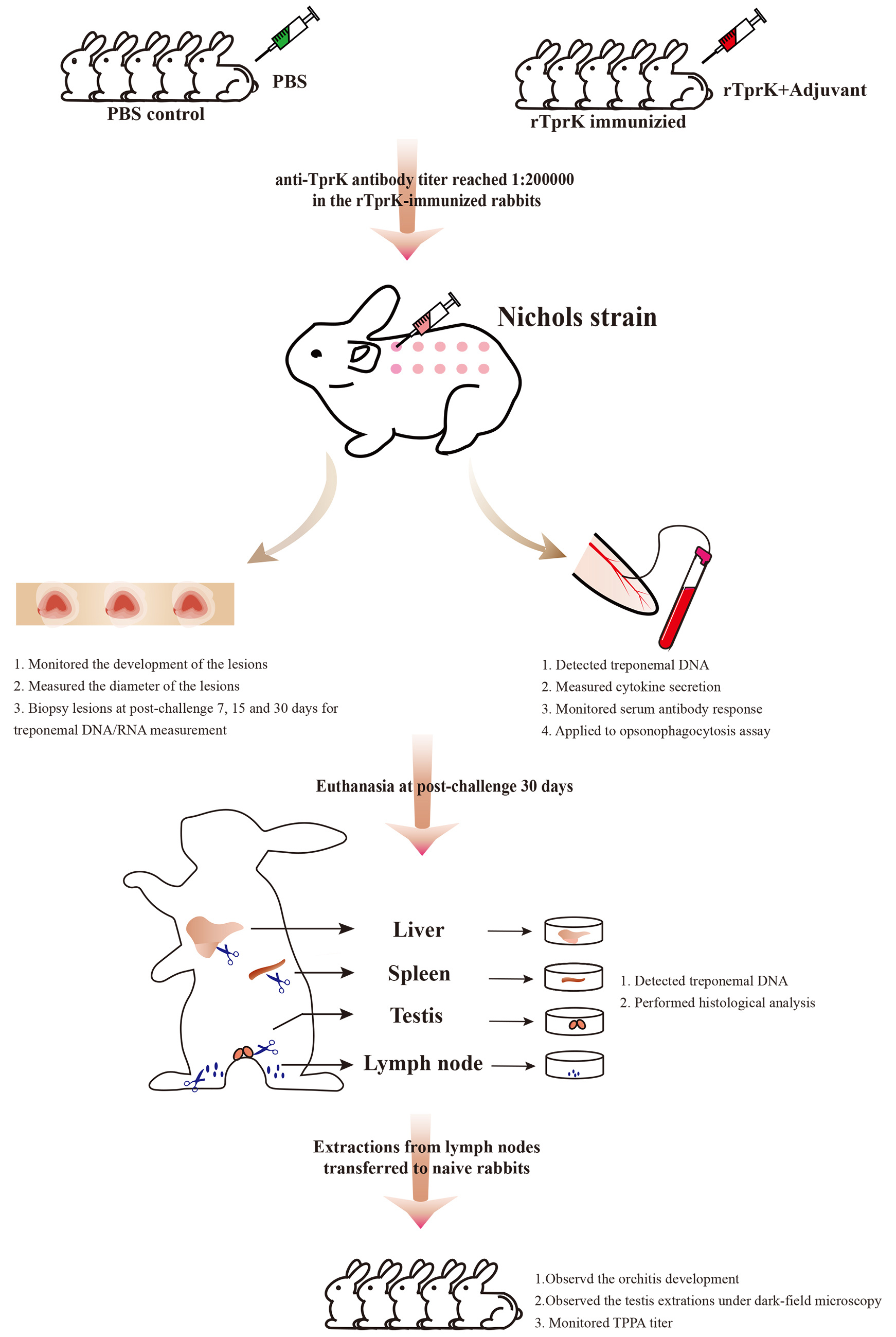
Figure 1. Overview of the immunization and challenge infection procedure
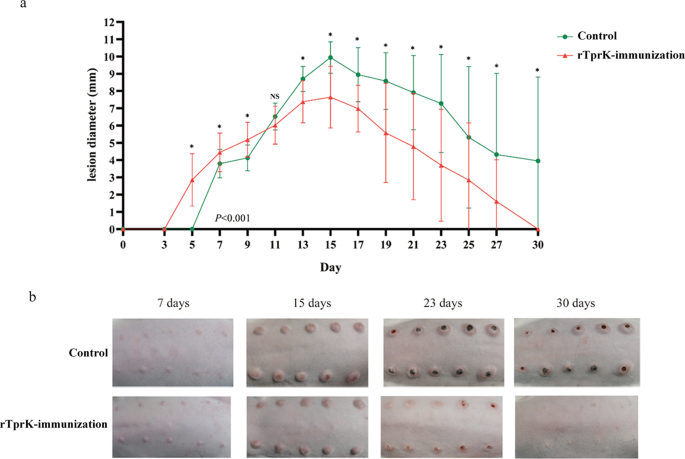
In this study, obvious attenuation of lesion development and lower T. pallidum load in the tissue sites were observed in the rTprK-immunized rabbits, which supported the results of Centurion-Lara’s. Besides, we found that immunization with rTprK elicited a robust Th1-cell response led by high expression of IFN-γ. Based on the examination of histopathology of primary lesions in human syphilis infection as well as rabbit infection models, the production of Th1-skewed cytokines to induce a strong DTH response was critical for limiting the number of T. pallidum10-12. In the study, within 7 days postchallenge, a typical DTH response was observed in the rTprK-immunized rabbits. Meanwhile, the levels of the Th1-like cytokines IFN-γ, TNF-α, and IL-12 were significantly increased in the sera of rTprK-immunized rabbits, and at 7 days postchallenge, the secretion of these cytokines peaked. IFN-γ is a marker of the DTH response, and the significantly increased expression of this marker corroborated the occurrence of a typical DTH response in the rTprK-immunized rabbits. There was also some indirect evidence of abundant immune cell infiltration in the spleen of the rTprK-immunized rabbits, suggesting that the cellular immune response in the immunized rabbits was intense. In combination with the significantly reduced number of T. pallidum in lesions of the immunized rabbits, the results indicated that immunized rTprK could rapidly evoke the host to robustly activate the Th1 immune response to limit the number of T. pallidum in lesions, thus altering lesion development.
In addition, the humoral response induced by rTprK in the presence of opsonic antibodies enhanced macrophage-mediated opsonophagocytosis. Prior to this, whether TprK is a target of the opsonic antibodies has been a subject of debate between the two laboratories. Given the current diversity and sophistication of research tools, we applied flow cytometry analysis and indirect immunofluorescence microscopy to analyze the role of anti-rTprK sera. The opsonophagocytosis results showed that macrophage-mediated opsonization of T. pallidum with prechallenge sera from the rTprK-immunized rabbits led to a higher phagocytosis rate. Furthermore, with postchallenge sera from the immunized rabbits, macrophage-mediated opsonization of T. pallidum was significantly strengthened. The findings proved that antibodies against rTprK could enhance opsonophagocytosis of T. pallidum.
Of course, there are some shortcomings that we have to admit. Because of the use of the rabbit models, the number of kits suitable for inflammatory detection was limited so the exploration of Th1-cell responses is insufficient. Based on this, we have discussed the possibility of using a mouse model instead of a rabbit model. The most important problem with using a mouse model is that there was no apparent ulceration and the immune response was not intense. Exploring the difference in the immune response between the rabbit and the mouse may be the first thing to resolve before using a mouse model instead of a rabbit model. More importantly, the study did not further investigate the epitopes directed to the T-cell response and B-cell response, which would shed light on the development of effective measures to cope with clinical T. pallidum strains with highly variable tprK genes. To investigate the epitopes of Nichols tprK gene which is directed to the T-cell response would be the next experiment plan.
Reference
- Li, W., Li, Q.L., Xu, Q.Y., Wang, X.T. & Yang, T.C. Tp47 promoted the phagocytosis of HMC3 cells though autophagy induced by endoplasmic reticulum stress. Journal of the European Academy of Dermatology and Venereology : JEADV 36, 2224-2234 (2022).
- Edmondson, D.G., Hu, B. & Norris, S.J. Long-Term In Vitro Culture of the Syphilis Spirochete Treponema pallidum subsp. pallidum. mBio 9, e01153-01118 (2018).
- Kojima, N., Konda, K.A. & Klausner, J.D. Notes on syphilis vaccine development. Frontiers in immunology 13(2022).
- Radolf, J. D. & Kumar, S. The Treponema pallidum Outer Membrane. Current Topics in Microbiology and Immunology 415, 1-38 (2018).
- Hazlett, K.R.O., et al. The Tprk Protein of Treponema pallidum Is Periplasmic and Is Not a Target of Opsonic Antibody or Protective Immunity. The Journal of experimental medicine 193, 1015-1026 (2001).
- Centurion-Lara, A., et al. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. The Journal of experimental medicine 189, 647-656 (1999).
- Liu, D., et al. Profile of the tprK gene in primary syphilis patients based on next-generation sequencing. PLoS neglected tropical diseases 13, e0006855 (2019).
- Stamm, L.V. & Bergen, H.L. The sequence-variable, single-copy tprK gene of Treponema pallidum Nichols strain UNC and Street strain 14 encodes heterogeneous TprK proteins. Infection and Immunity 68, 6482-6486 (2000).
- Lukehart, S.A. & Marra, C.M. Isolation and laboratory maintenance of Treponema pallidum. Current protocols in microbiology Chapter 12, Unit 12A 11 (2007).
- LaFond, R.E. & Lukehart, S.A. Biological basis for syphilis. Clinical Microbiology Reviews 19, 29 (2006).
- Arroll, T.W., Centurion-Lara, A., Lukehart, S.A. & Voorhis, W.C.V. T-Cell Responses to Treponema pallidum subsp. pallidum Antigens during the Course of Experimental Syphilis Infection. Infection and Immunity 67, 4757-4763 (1999).
- Sell, S., Gamboa, D., Baker-Zander, S.A., Lukehart, S.A. & Miller, J.N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. The Journal of investigative dermatology 75, 470-475 (1980).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in