It’s time to give a crap about preserving fecal samples
Published in Microbiology

Over the last decade, research studies have connected almost any disease you can name to the gut microbiome. As a result, there is now widespread interest in using the gut microbiome as a target for developing new therapeutics and diagnostics. Fundamental to microbiome research though, is the assumption that the method used to collect fecal samples is not distorting the microbial community. If this assumption is incorrect, researchers can chase false leads and miss key biomarkers.
We didn’t begin with the intention of undertaking a study to assess fecal preservation methods; our main goal was to precisely measure the gut microbiome using shotgun metagenomics. However, when we started researching various room temperature preservation methods, we were astonished that few studies had assessed these methods using shotgun metagenomic sequencing1–4, and of those studies, only one had assessed technical reproducibility4. We realized that to make a well-informed decision, we would need to undertake our own study.
We chose five different methods to evaluate, including three methods commonly used in faecal microbiome studies: OMNIgene-GUT, a dry BBL CultureSwab, and RNAlater; as well as two methods designed for different sample types, but that we thought had good potential for preserving microbial DNA: LifeGuard – a preservative solution primarily for soil samples, and the FLOQSwab in an active drying tube (FLOQSwab-ADT) – primarily used for forensic DNA sampling. To rigorously assess the technical and compositional reproducibility of each method, we used six replicates from five different individuals and compared all methods to the commonly accepted best practice of flash freezing (Fig 1). All samples were analysed using shotgun metagenomics, for a total of 180 metagenomes.
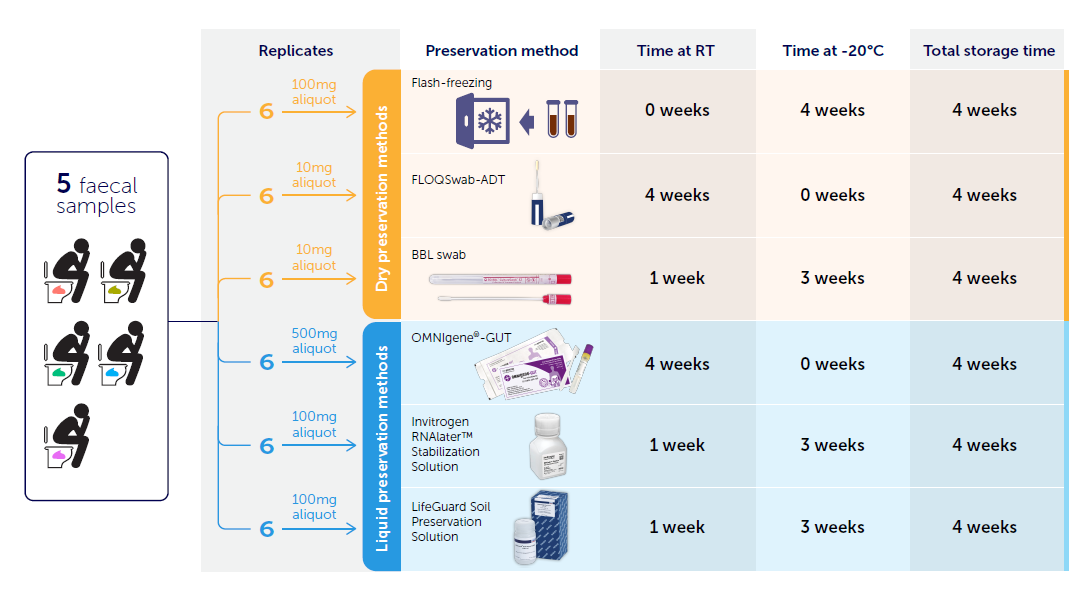
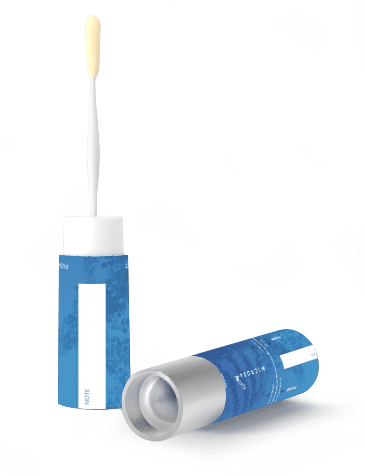
We were surprised to find that the overall best performing method was one that had not been previously used in faecal microbiome studies, the FLOQSwab-ADT. This is a liquid-free, swab-based method that preserves DNA by desiccating it (Fig 2). Concerningly, we also found that both the BBL CultureSwab and LifeGuard allowed for the growth of facultatively anaerobic species such as Escherichia coli in a non-predictable manner among replicates.
To determine the range of use of the FLOQSwab-ADT, we further evaluated it in an additional ten individuals at various storage temperatures (-20ᵒC, room temperature and 50ᵒC), for a total of 240 metagenomes. The FLOQSwab-ADT was able to accurately reproduce microbial communities at all temperatures when stored for up to four weeks.
Through rigorous evaluation, we identified the FLOQSwab-ADT as the best option for use in gut microbiome studies, especially where faecal samples need to be collected and transported across long distances. To our knowledge, this is the most comprehensive study to assess both the technical and compositional reproducibility of this number of room temperature preservation methods using shotgun metagenomic sequencing.
Read the full article at ISME Communications - Critical evaluation of faecal microbiome preservation using metagenomic analysis.
- Anderson, E. L. et al. A robust ambient temperature collection and stabilization strategy: Enabling worldwide functional studies of the human microbiome. Sci. Rep. 6, 31731 (2016).
- Byrd, D. A. et al. Reproducibility, stability, and accuracy of microbial profiles by fecal sample collection method in three distinct populations. PLOS ONE 14, e0224757 (2019).
- Franzosa, E. A. et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. 111, E2329 (2014).
- Voigt, A. Y. et al. Temporal and technical variability of human gut metagenomes. Genome Biol. 16, 73 (2015).





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in