Keep calm and monitor BAM: How the lipoprotein RcsF senses stress by monitoring the BAM machinery
Published in Microbiology
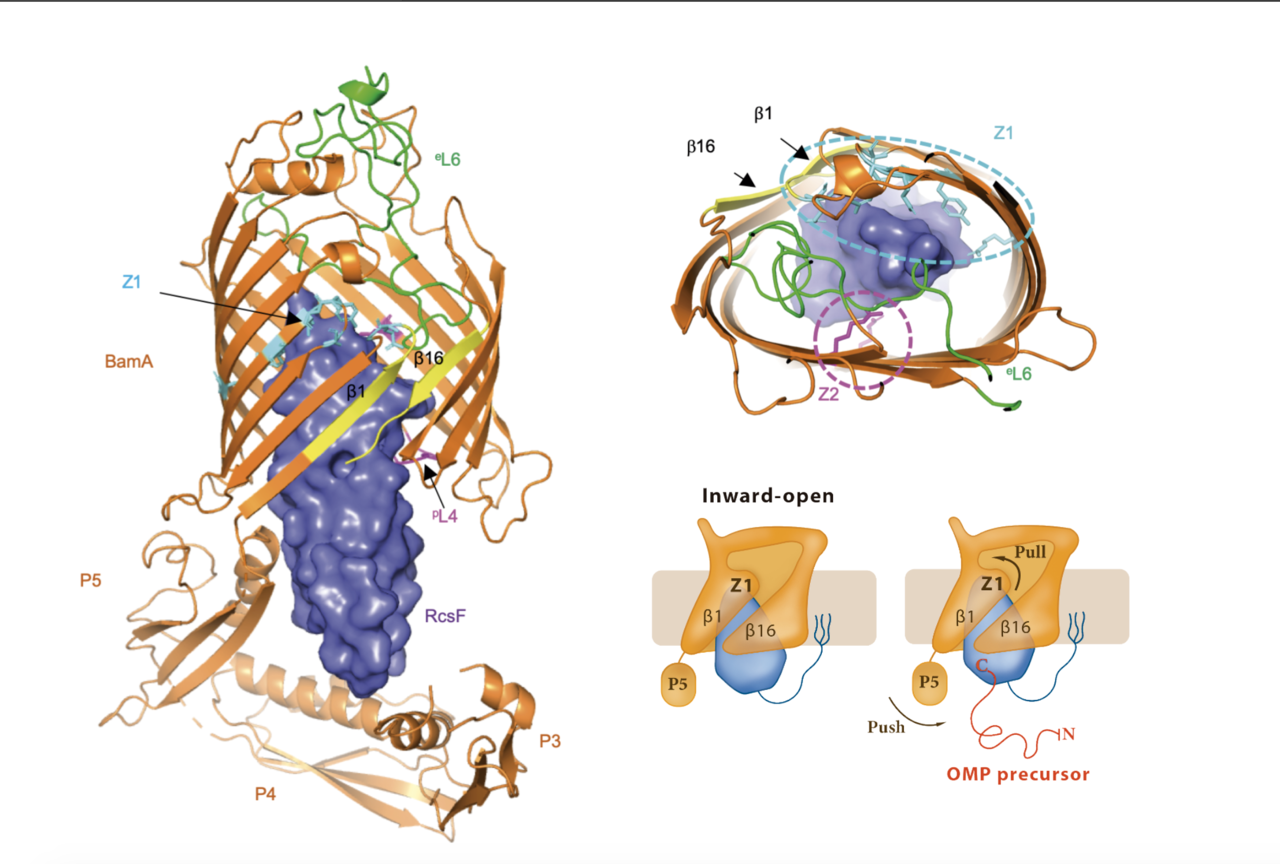
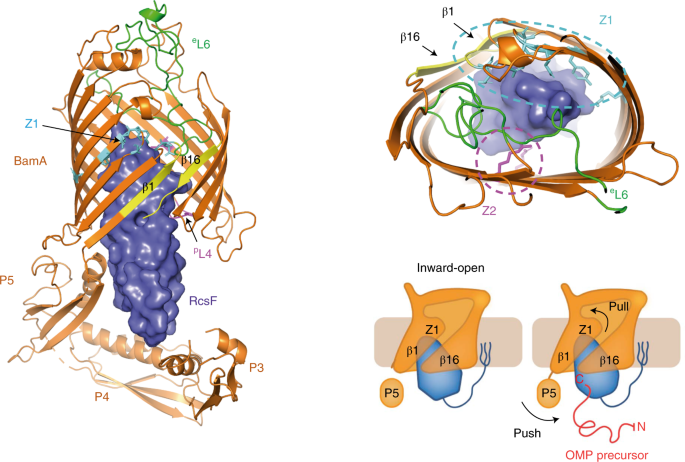
The β-barrel assembly machinery (BAM) inserts outer membrane β-barrel proteins (OMPs) in the outer membrane of Gram-negative bacteria. In Enterobacteriaceae, BAM also mediates the export of the stress sensor lipoprotein RcsF to the cell surface by assembling RcsF-OMP complexes (1,2).
Intrigued by the interaction between RcsF and BamA we started working in the crystallization of the complex. To help us at this point we enlisted the help of a structural biologist specialist Dr. Han Remaut at VUB. Together we obtained the crystal structure of RcsF in complex with BamA, the first structure of BamA in complex with a lipoprotein. RcsF is lodged deep inside the lumen of BamA β-barrel. This interaction is only possible as long as BamA β-barrel remains in a lateral close conformation. By showing that the globular domain of RcsF is lodged deep inside the barrel of BamA, our structure also reveals the remarkable—and unanticipated —finding that the BamA β-barrel can accommodate a lipoprotein “substrate” with a globular domain of 12 kDa in size. Moreover, we proposed a push and pull mechanism model for the export of RcsF. This mechanism involves the conformational cycling of BamA and provides a mechanistic explanation for how RcsF uses its interaction with BamA to detect envelope stress.
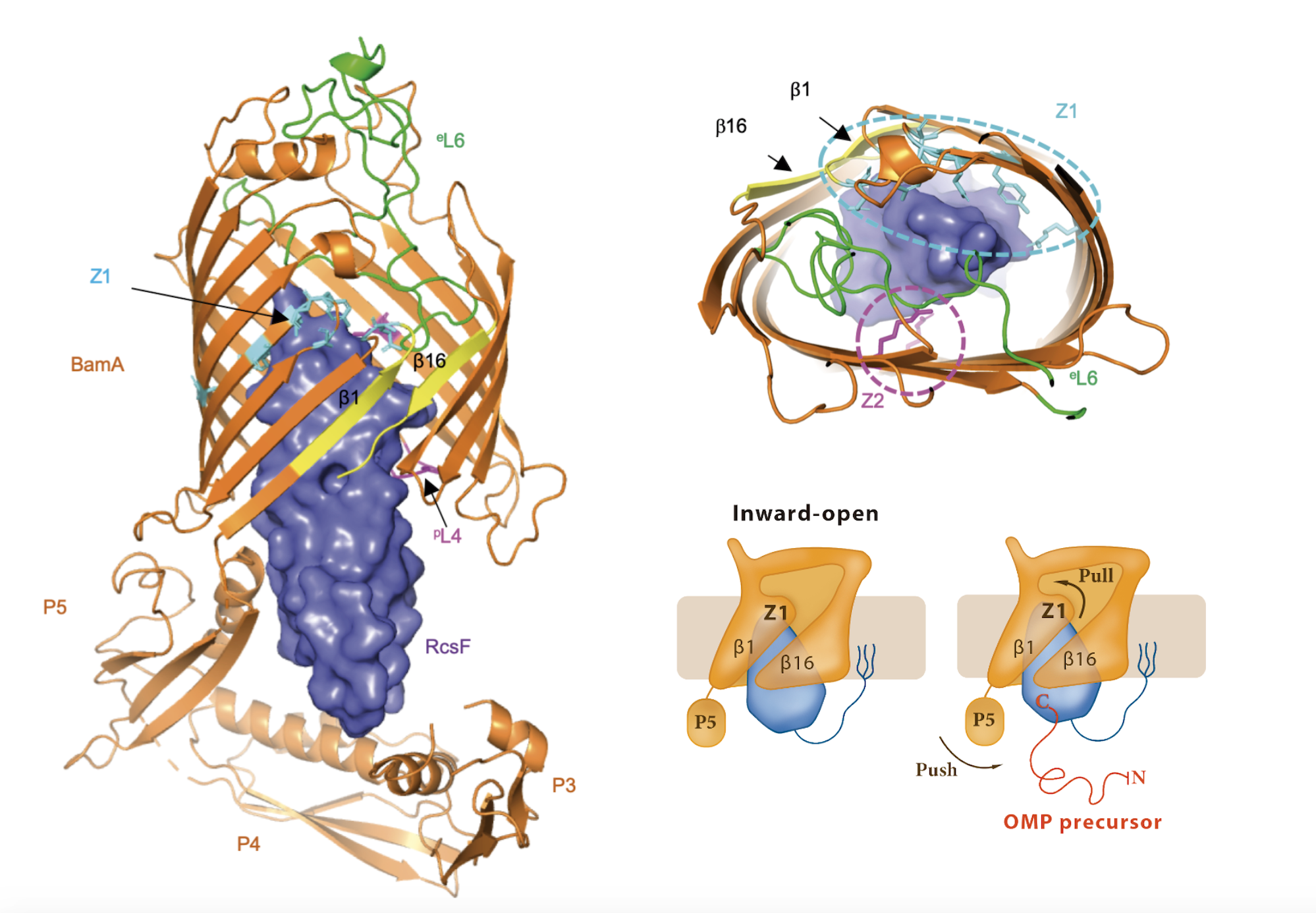
This finding further establishes BAM as an essential hub that contributes to outer membrane biogenesis by interacting both with nascent OMPs for assembly and lipoproteins for export. Future work will reveal whether other lipoproteins bind BamA in a way similar to RcsF.
In the following link you can find the Dynamic Importance Sampling (DIMS) simulation of the BamA-RcsF complex reproducing the proposed push-and-pull model performed by our collaborator Dr. Bogdan I. Iorga. The simulation shows the transition of BamA from the inward-open to the outward-open conformation, with the POTRA5 domain moving towards the periplasmic exit of the lumen and pushing RcsF upwards. This movement is accompanied later on by the movement of strands 1-6 in BamA and the opening of the outward-facing extremity. The initial conformation of the system (BamA and RcsF) corresponds to the inward-open structure determined in this work (PDB code 6T1W) with the POTRA1-4 domains removed. The final conformation of BamA is similar to the outward-open structure (PDB code 5D0Q). The proteins are represented as cartoons (BamA and RcsF colored in orange and blue, respectively), the explicit outer membrane represented as sticks, and the oxygen atoms of water molecules represented as red dots.
You can read the full article at Nat Chem Biol under the title of “Structural insight into the formation of lipoprotein-β-barrel complexes”
https://www.nature.com/articles/s41589-020-0575-0
1. Cho, S. H.et al. Detecting Envelope Stress by Monitoring β-Barrel Assembly. Cell159, 1652-1664, DOI:10.1016/j.cell.2014.11.045 (2014).
2. Konovalova, A., Perlman, D. H., Cowles, C. E. & Silhavy, T. J. Transmembrane domain of surface-exposed outer membrane lipoprotein RcsF is threaded through the lumen of beta-barrel proteins.Proc Natl Acad Sci U S A111, E4350-4358, DOI:10.1073/pnas.1417138111 (2014).
Follow the Topic
-
Nature Chemical Biology

An international monthly journal that provides a high-visibility forum for the chemical biology community, combining the scientific ideas and approaches of chemistry, biology and allied disciplines to understand and manipulate biological systems with molecular precision.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in