Lab-scale UV-Vis Spectroscopy Monitors Oxidation State Changes in Materials for Energy Storage
Published in Materials
Developing new batteries or supercapacitors that can meet various power and energy density requirements is crucial for advancing energy technology. To achieve this, we need to understand the different ways that energy is stored in electrochemical energy storage devices. There are a variety of techniques used to distinguish different mechanisms of energy storage processes, for example, by running cyclic voltammetry and various operando/in situ characterization methods such as Raman spectroscopy, X-ray absorption spectroscopy, X-ray diffraction, electron energy loss spectroscopy in the transmission electron microscope, etc. However, each technique has its own limitations, such as the requirement of expensive instrumentation, hard-to-access synchrotron light sources, or specialized data processing.
Beyond the above techniques, spectroelectrochemistry, which involves electrochemistry coupled with optical spectroscopy techniques attracted much attention. The related studies demonstrated in situ UV-Vis spectroscopy as a powerful tool for studying charge storage mechanisms. However, the existing studies mainly focused on using optical spectroscopy to investigate specific charge storage mechanisms, such as how and where the ions are stored in materials. Herein, we expanded the use of UV-Vis spectroscopy as a generic method for distinguishing intercalation-based battery-type, surface redox, and double-layer charge storage processes. Moreover, this technique can provide detailed quantitative insights into the charge storage mechanisms and oxidation state changes.
In our article In Situ Monitoring Redox Processes in Energy Storage Using UV-Vis Spectroscopy in Nature Energy (https://rdcu.be/c9kEA), we investigated Ti3C2Tx MXene in neutral aqueous electrolyte for electrical double-layer and in sulfuric acid for pseudocapacitance, and lithium titanate (LTO) in a lithium perchlorate in acetonitrile electrolyte for battery-type redox mechanisms. We used two methods to collect UV-Vis absorption data. One applied constant potential step by step to the device, waited to reach the equilibrium state, and collected the full spectra in the UV-Vis range. The other involved running cyclic voltammetry (CV) while collecting the absorption at certain fixed wavelengths. Figure 1 shows optical CVs (blue curves) derived from UV-Vis data matched electrochemical CVs (red curves). This illustrates that the UV-Vis absorbance change of electrode material is highly responsive to the change in the state of charge, making UV-Vis spectroscopy a promising technique for studying redox processes in energy storage systems.
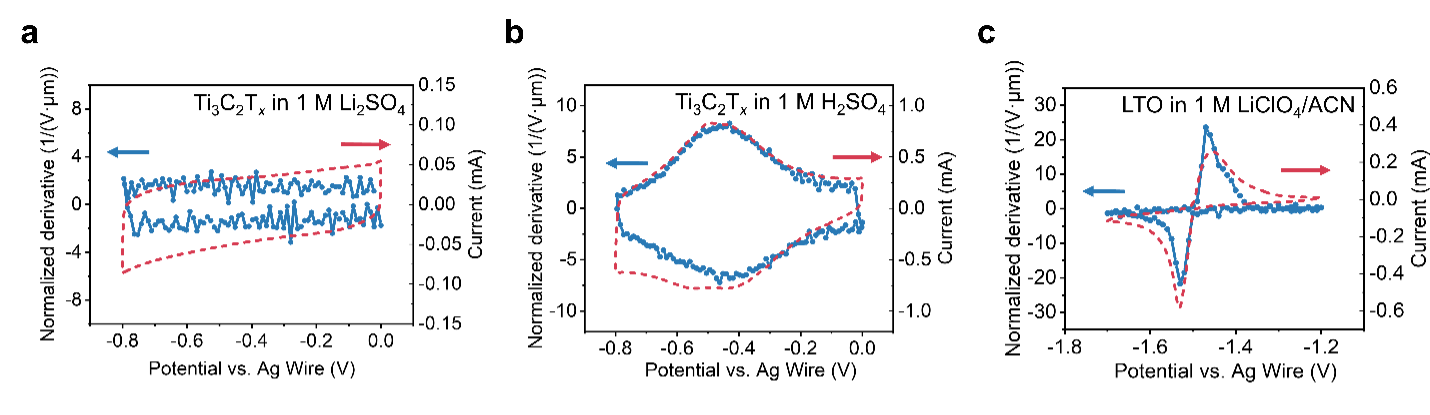 Figure 1. Comparison of electrochemical and UV-Vis cyclic voltammetry (CV) curves of selected electrochemical systems collected using the CV method for three electrochemical systems: a, double-layer, b, pseudocapacitive, and c, battery-type redox.
Figure 1. Comparison of electrochemical and UV-Vis cyclic voltammetry (CV) curves of selected electrochemical systems collected using the CV method for three electrochemical systems: a, double-layer, b, pseudocapacitive, and c, battery-type redox.
Then we extrapolate this method to other systems like Prussian Blue (PB) in potassium chloride and MXene in water-in-salt electrolyte to further compare different energy storage processes. We demonstrated that Ti3C2Tx MXene behaves like a double-layer capacitor in a water-in-salt system. In general, battery-type systems have steeper slopes, as seen in the purple and orange lines in Figure 2a. Double-layer systems (green curves) have gentler slopes, while the pseudocapacitive system (black curve) falls in the middle. By calculating the derivative ratio from the optical CVs, one can determine which kind of energy storage mechanism is compared to others for a given system, as shown in Figure 2b.
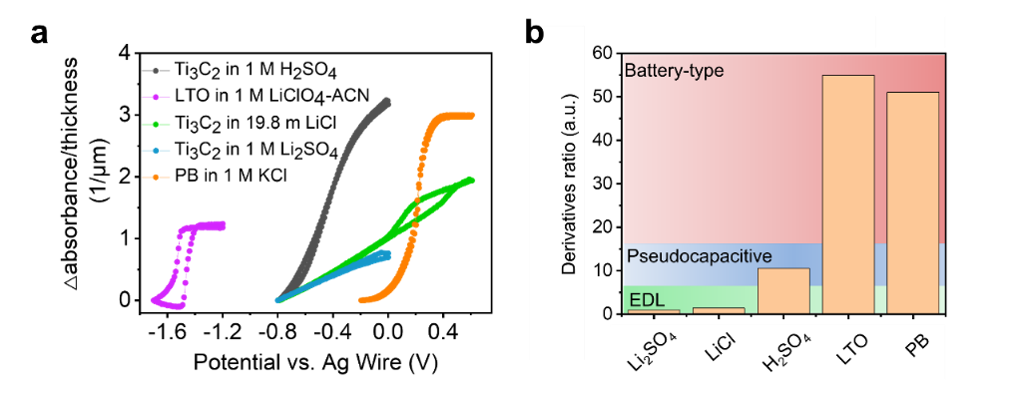
Figure 2. The distinction of charge storage mechanisms with the slope of relative absorbance change and normalized derivative. a, Relative absorbance change in the UV-Vis absorption spectra at the signature wavelength. b,Distinguishing electrical double-layer, pseudocapacitive, and battery-type charge storage mechanisms by comparing derivative ratios.
Furthermore, by combining Beer-Lambert’s law and Faradaic law of electrolysis, a quantitative correlation between optical information and electrochemical information was established to calculate the estimated electron transfer number in the pseudocapacitive system, and the number is close to the results from in situ X-ray absorption measurements.
We have demonstrated that in situ UV-Vis spectroscopy is a powerful technique for determining charge storage mechanisms and monitoring redox processes in different electrochemical systems. Compared to conventional methods, UV-Vis spectroscopy is affordable, accessible, fast, and non-destructive. We envision that UV-Vis will play an increasingly important role in in situ studies of a wide range of electrochemical phenomena in materials, ranging from energy storage to SEI formation, electrolyte decomposition, electrocatalysis, electrochromism, and electrochemical modulation of materials properties. https://www.nature.com/articles/s41560-023-01240-9.epdf
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in