Legionella Dot/Icm type IV secretion system: A molecular machine gun
Published in Microbiology
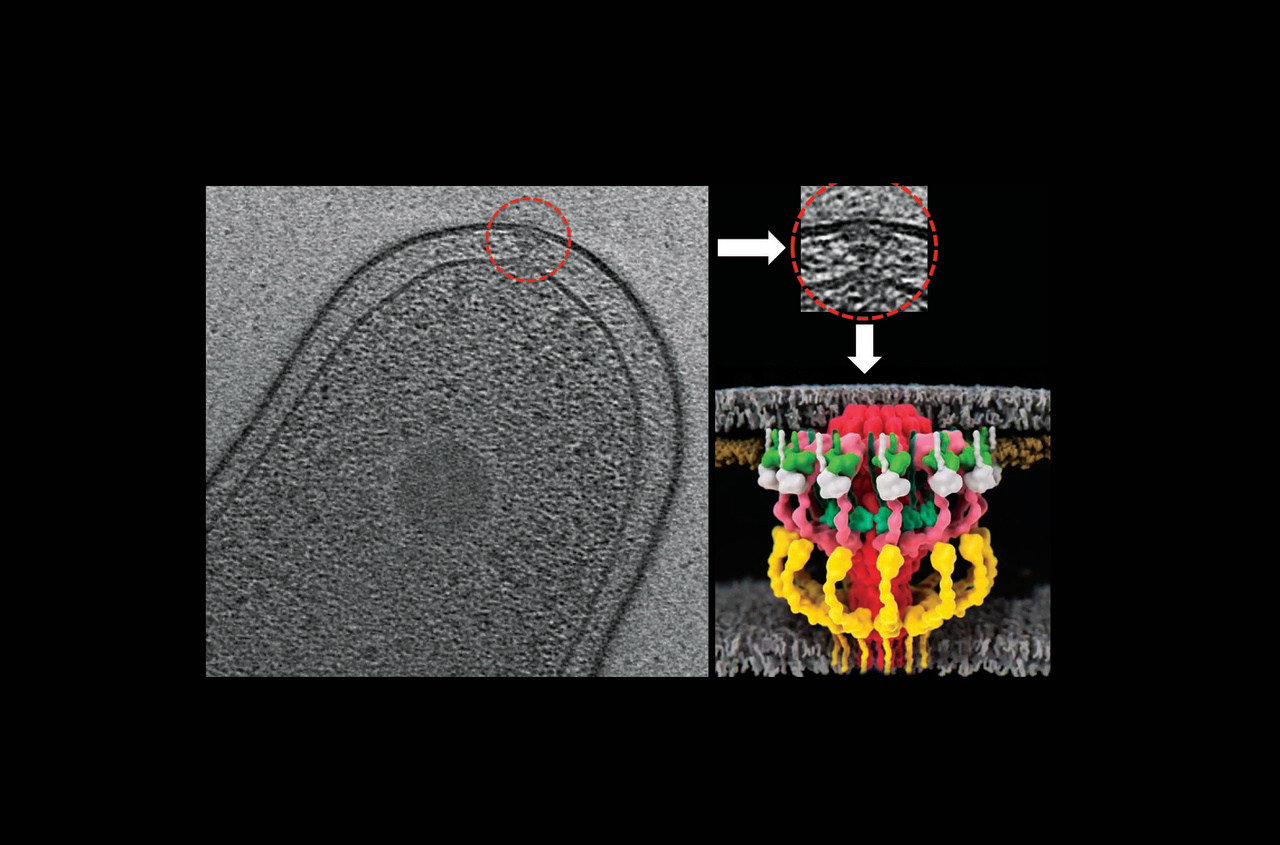
Type IV secretion systems (T4SS) are widely present molecular machines that translocate a broad range of macromolecules (e.g. proteins, nucleoprotein complexes etc.) across kingdom barriers [1,2]. The Legionella pneumophila Dot/Icm T4SS has received a significant amount of attention of late as this specialized nanomachine has more than twice the number of components as canonical conjugative T4SSs and secretes an astounding number of effector proteins (300+) into host cells to cause Legionnaires’ disease [3]. This system was co-discovered in Ralph R. Isberg’s lab at Tufts University and Howard Shuman’s lab at Columbia University [4,5].
Joseph Vogel, a former postdoctoral fellow in the Isberg lab, continued working on this amazing molecular machine in his own lab at the Washington University School of Medicine in St. Louis, MO. Using extensive genetic, biochemical and immunofluorescence microscopy (IFM) analyses, Dr. Vogel’s lab deciphered the localization and many of the molecular connectivities between components of this 27-protein secretion system [6–8]. However, the overall molecular architecture of this multi-megadalton complex remained elusive. Attempts to purify (or reconstitute) and solve the structure of this molecular machine were unsuccessful due to its complexity, flexibility and tight integration within all three layers of the Gram-negative bacterial cell envelope.
Meanwhile, Grant Jensen’s lab at Caltech has been imaging complex molecular machines within bacterial cells (e.g. cytoskeletal elements, secretion systems etc.) and solving their in situ structures using state-of-the art electron cryotomography (ECT) and subtomogram averaging methods [9,10]. When I joined Caltech as a postdoctoral fellow, Grant Jensen’s laboratory was looking for an amenable system to reveal the first in situ structure of a bacterial T4SS. This resulted in a very complementary and successful collaboration between the Vogel and Jensen laboratories. In 2017, using ECT and subtomogram averaging method, we revealed the first in situ structure of the L. pneumophila Dot/Icm T4SS with all its components at about 2-4 nm resolution [11]. Our work showed that despite the vastly different genetic organization, the conjugative T4SS and the Legionella Dot/Icm T4SS are architecturally similar. However, at this resolution, it was difficult to dissect the molecular organization of this complex.
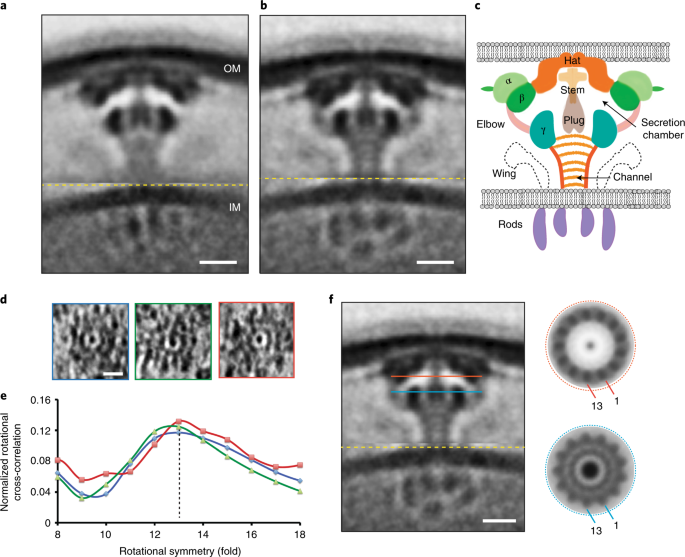
Previously the Vogel lab had constructed individual deletions in every component of the Legionella Dot/Icm T4SS. By imaging many of those strains and a number of rationally designed fusions, we discovered in this manuscript the molecular organization and connectivities of a large portion of the Legionella Dot/Icm T4SS. We imaged 17 different strains, in which certain components were either lacking or fused to a tag, collected ~2300 tomograms and generated 11 new averages. We then calculated difference maps with respect to the wild type structure. All these data together allowed us to put together an elaborate 3-D jigsaw puzzle, thereby locating all of the outer membrane, periplasmic, and inner membrane components with significant periplasmic domains. Additionally, we presented a higher-resolution average of the Dot/Icm T4SS complex revealing many interesting structural details and mechanistic insights. We posted this manuscript (Ghosal et al) on bioRxiv (https://www.biorxiv.org/content/10.1101/312009v1) in May 2018.
Independent of this analysis, the Vogel lab had shown that the Dot/Icm complex is present at both poles of the bacterial cells and polar secretion is required for the proper intracellular targeting of the L. pneumophila containing vacuole [8]. Using a combination of genetic manipulation, IFM and biochemical analyses, they meticulously identified the polar targeting factors for the Dot/Icm T4SS and determined the interdependencies between many of the Dot/Icm components in the initial steps of assembly of this T4SS. This study (Jeong et al) was independently posted on bioRxiv (https://www.biorxiv.org/content/10.1101/315721v1) in May 2018. Because both studies were remarkably complementary and supported the conclusions of one another, we decided to merge the two manuscripts into one over-encompassing manuscript and submit to Nature Microbiology. Due to space limitation, many of our exciting results could not be placed in the main text and thus we would encourage readers of this combined manuscript (Ghosal* and Jeong* et al) to view the numerous supplementary figures.
In a complementary manuscript (Chetrit* and Hu* et al.) [12], Craig Roy and Jun Liu’s group combined IFM and ECT to decipher the molecular architecture of the cytoplasmic ATPase complex of the same molecular machine. They also presented a higher-resolution average of the Dot/Icm T4SS in situ and independently reported the presence of a central secretion channel and an unusual 13-fold symmetry for the outer membrane complex. We found that the average Chetrit* and Hu* et al reported for one of the strains, a ΔdotG mutant, was very different from what we saw and surprising given existing structural and biochemical data [11,13,14]. We obtained their strain, sequenced and re-imaged it, confirming our previous result (see Supplementary Figure 9 in our paper). Intriguingly, it is hard to reconcile their proposed ATPase complex structure with previous structural results and the expected multimerization properties of the Dot/Icm ATPases [12,13,15]. It will therefore be exciting to unravel this and other mysteries with further investigation.
Our manuscript (Ghosal* and Jeong* et al) in Nature Microbiology can be found here: https://www.nature.com/articles/s41564-019-0427-4
References:
1. Christie, P. J. & Vogel, J. P. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8, 354–360 (2000).
2. Chandran Darbari, V. & Waksman, G. Structural Biology of Bacterial Type IV Secretion Systems. Annu. Rev. Biochem. 84, 603–629 (2015).
3. Segal, G., Feldman, M. & Zusman, T. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol. Rev. 29, 65–81 (2005).
4. Vogel, J. P., Andrews, H. L., Wong, S. K. & Isberg, R. R. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279, 873–876 (1998).
5. Segal, G., Purcell, M. & Shuman, H. A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. U.S.A. 95, 1669–1674 (1998).
6. Vincent, C. D. et al. Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system. Mol. Microbiol. 62, 1278–1291 (2006).
7. Sutherland, M. C., Binder, K. A., Cualing, P. Y. & Vogel, J. P. Reassessing the role of DotF in the Legionella pneumophila type IV secretion system. PLoS ONE 8, e65529 (2013).
8. Jeong, K. C., Ghosal, D., Chang, Y.-W., Jensen, G. J. & Vogel, J. P. Polar delivery of Legionella type IV secretion system substrates is essential for virulence. Proc. Natl. Acad. Sci. U.S.A. 114, 8077–8082 (2017).
9. Oikonomou, C. M. & Jensen, G. J. Cellular Electron Cryotomography: Toward Structural Biology In Situ. Annu. Rev. Biochem. 86, 873–896 (2017).
10. Oikonomou, C. M. & Jensen, G. J. A new view into prokaryotic cell biology from electron cryotomography. Nature Reviews Microbiology 15, 128 (2017).
11. Ghosal, D., Chang, Y.-W., Jeong, K. C., Vogel, J. P. & Jensen, G. J. In situ structure of the Legionella Dot/Icm type IV secretion system by electron cryotomography. EMBO Rep. 18, 726–732 (2017).
12. Chetrit, D., Hu, B., Christie, P. J., Roy, C. R. & Liu, J. A unique cytoplasmic ATPase complex defines the Legionella pneumophila type IV secretion channel. Nat Microbiol 3, 678–686 (2018).
13. Low, H. H. et al. Structure of a type IV secretion system. Nature 508, 550–553 (2014).
14. Chandran, V. et al. Structure of the outer membrane complex of a type IV secretion system. Nature 462, 1011–1015 (2009).
15. Peña, A. et al. The hexameric structure of a conjugative VirB4 protein ATPase provides new insights for a functional and phylogenetic relationship with DNA translocases. J. Biol. Chem. 287, 39925–39932 (2012).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in