Liposomal vaccine that block the spreading of Malaria disease
Published in Bioengineering & Biotechnology
I joined the State University of New York at Buffalo in Dr. Jonathan Lovell’s lab in the summer of 2015, after carrying out liposome research at Academia Sinica in Taiwan with Dr. Steve Roffler. Prior to that, I obtained a MS degree in biomedical engineering from National Tsing Hua University in Taiwan. Malaria is caused by the plasmodium parasite, which is transmitted to human by mosquito bites. An effective malaria vaccine would prevent it from developing in an immunized individual, or alternatively could stop malaria from spreading from an infected individual to a new host. This latter approach, is transmission blocking, and it prevents parasite development in the mosquito midgut. Transmission-blocking vaccines have been proposed, but not yet tested in large clinical studies. Pfs25 is one of the most well-known transmission blocking antigens, but due to its poor immunogenicity, clinical trials have not been successful. In this work, we developed a liposomal vaccine adjuvant, that contained two active lipids: PHAD (synthetic Monophosphoryl lipid A) and cobalt porphyrin-phospholipid (CoPoP). CoPoP has been shown to have high binding affinity for proteins that have a polyhistidine tag. These liposomes can be mixed with his-tagged proteins for spontaneous nanoliposome antigen particleization (SNAP). We used the SNAP approach with Pfs25 and found promising results. This project was carried out in collaboration with PATH-MVI, who helped guide us through the research and experimental design, as we did not have experience with malaria when we started the project.
To characterize SNAP, his-tagged Pfs25 was mixed with CoPoP liposomes, then we used a filtration assay to separate free and liposome-bound Pfs25 in the mixture. Later, we used an immunoprecipitation assay to confirm that the conformation of Pfs25 is intact after it binds the liposomes. To process this experiment, protein G beads were coated with anti-Pfs25 antibodies, and incubated with Pfs25 that had been particleized with the liposomes. A challenge of the immunoprecipitation assay was that the amount of antigen to incubate with the antibody-coated beads had to be determined precisely; if we add too much liposome-bound antigen, then the beads would not be able to bound to pull down all the liposomes. By including a control group with beads coated with non-specific antibody as a negative control, conditions could be optimized. The results show that CoPoP can particleize Pfs25 without disruption its confirmation Later, we moved into in vivo studies, and mice were immunized with different doses of Pfs25 with CoPoP liposomes. The initial results were startling; since when we immunized mice with 100 ng of liposome-bound Pfs25, we observed a potent antibody titer compared to the free antigen. I remember Dr. Lovell’s reaction when saw the data, as he said “Amazing…Unbelievable…We need to repeat this!” with eyes that sparkled with excitement. Soon, we confirmed that his-tagged Pfs25 is really working like magic when combined with CoPoP liposomes. Most other commercial vaccine adjuvant were not able to induce functional antibody with Pfs25 at the low doses we used.
One of the challenges of this project was to gain an better understanding of why SNAP works so well as an adjuvant for Pfs25. We investigated the uptake of antigen draining lymph nodes to antigen presenting cells. I faced some difficulties in collecting the draining lymph node from the mice. I asked for help from my committee member Dr. Elizabeth Wohlfert, who is in the department of microbiology and immunology. Thankfully, one of her students taught me how to isolate the lymph node, and after practice, it became easy to find it. The lymph node study shows that high uptake of Pfs25 to antigen presenting cells only occurred when it was particleized with SNAP, and other adjuvants did not result in good antigen uptake. This points to a potential mechanism of the adjuvant efficacy.
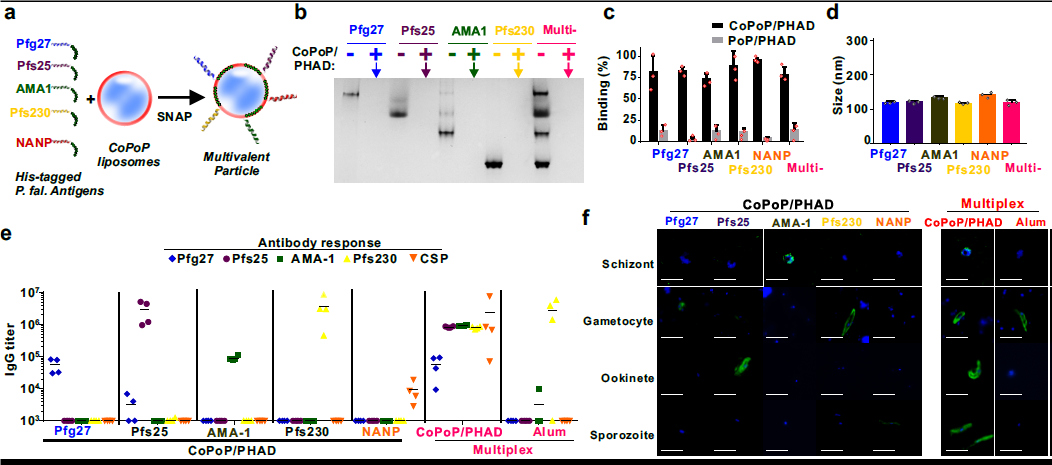
Another interesting result in our paper showed that malaria vaccine antigen that target different stages of the parasite lifecycle could easily bind to CoPoP liposomes for a multiplexed vaccine approach (Fig 1). We confirmed that the multiplex vaccine could induced specific antibody against different stage of parasites. To do so, we used a technique called immunofluorescence assay. We were able to obtain the parasites from Dr. Abhai Tripathi and Godfrey Mlambo at the Johns Hopkins malaria core facility. They were extremely helpful in providing us with the parasites at different life-stages. Although there was one minor and memorable hiccup during a key experiment: Something went wrong with Fedex shipping the sporozoite parasite samples from Hopkins to Buffalo. Since it takes several weeks to prepare them, and we were pressed for time, my colleague and co-author Cuiyan Lin and I decided to go a last-minute overnight road trip to Baltimore to get the samples. In the end, it was great seeing the parasitology core facility at Hopkins and meeting Dr. Mlambo, who was very kind and patient in showing us their techniques for isolating and fixing parasites.
Overall, because of the simplicity of SNAP, that operates by simple mixing of antigen and CoPoP liposomes without any further purification steps, I hope this approach becomes used for vaccine development of both single and multiplex antigen vaccines. I believe that SNAP can become a useful tool for researchers to develop vaccines to fight against malaria and also other diseases.
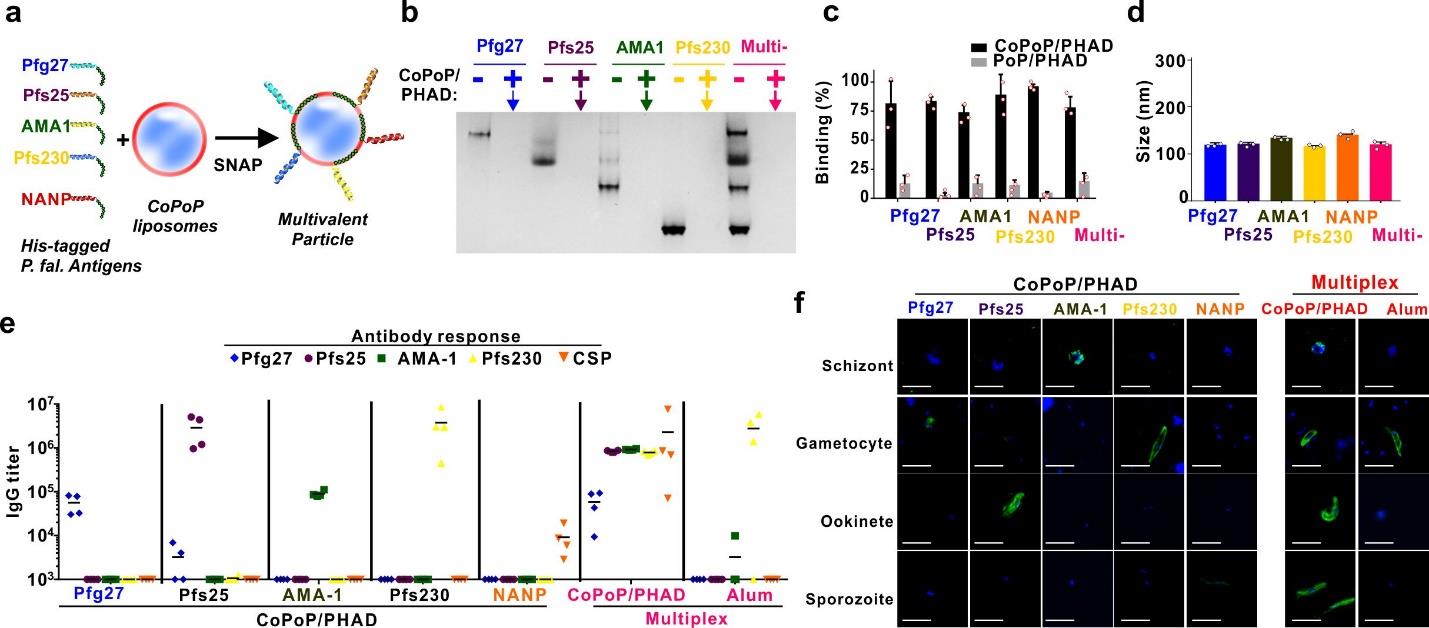
Multiplexed SNAP with P. falciparum antigens. a, Schematic representation of multivalent SNAP. b, Native PAGE gel demonstrating effective single and multiplexed antigen binding to CoPoP/PHAD liposomes. The absence of a protein band indicated by the arrow reflects antigen binding to the liposomes, which are too large to enter the gel. c, Single and multiplexed antigen binding as assessed by microcentrifugal filtration. d, Size of liposomes following SNAP. e, ELISA against the indicated antigens in mice immunized with individual antigens or multiplexed combination of all antigens. Lines show geometric means for n=4 mice per group. f, Immunofluorescence assay of indicated post-immune sera with fixed parasites. Bar: 10 μm. For c and d, error bars show mean +/- std. dev. for n=3 independent experiments. b and f show representative results of 3 independent experiments.
Our paper: Huang, W. C., B. Deng, C. Lin, K. A. Carter, J. Geng, A. Razi, X. He, U. Chitgupi, J. Federizon, B. Sun, C. A. Long, J. Ortega, S. Dutta, C. R. King, K. Miura, S. M. Lee and J. F. Lovell (2018).A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat Nanotechnol.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in