Making Superior Chemically Inert Low-Dimensional Interfaces Accessible for Perovskites
Published in Chemistry, Materials, and Sustainability
A low-dimensional (LD) interface layer – composed of a metal cation, an organic bulky cation, and a halide anion – has now become essential for enhancing the stability of perovskite solar cells without compromising their efficiency. In our earlier study (Nature Energy 2023, https://doi.org/10.1038/s41560-023-01204-z), we developed a full-precursor (FP) strategy, where both the metal and organic bulky cations are dissolved in acetonitrile – an optimized orthogonal solvent for perovskites. This approach allowed us to move beyond the conventional Pb2+/Sn2+ systems and explore a wider range of metal cations for LD interface design.
However, when we extended our FP strategy to explore organic bulky cations with a broader range of molecular structures, we encountered a new challenge: several LD materials formed with specific cations – such as (PiEA)PbI4 (PiEA = 2-piperidin-1-ium-1-ylethylammonium) – exhibited extremely poor solubility in acetonitrile (<1 mM), the very solvent that underpins the FP approach. This limitation made it impossible to construct these particular LD interfaces on perovskites using the FP strategy.
We then turned to the conventional half-precursor (HP) strategy, which constructs LD interfaces by depositing organic bulky cations onto perovskite films and allowing them to react in situ. However, this approach also failed. We found that specific bulky cations were chemically inert and showed no appreciable reactivity with the perovskite surface, making it impossible to directly form these LD interfaces through the HP route.
Rather than discouraging us, these limitations piqued our curiosity instead. What unique interfacial properties might these chemically inert LD interfaces offer if successfully integrated onto perovskites? And most importantly, how could we construct such interfaces despite their solubility and reactivity constraints?
Motivated by these questions, we set out to explore an alternative approach to construct these chemically inert LD interfaces. During a brainstorming session, we speculated whether a conventional 2D layer could be grown first using the FP strategy, followed by the deposition of these chemically inert organic bulky cations to trigger an organic cation exchange. This simple idea led to a real ‘eureka’ moment – and the conception of our selective templating growth (STG) strategy. Remarkably, it worked.
After a series of experiments and a fair bit of trial and error, we found that the conventional 2D phase PA2PbI4 (PA = phenylammonium) served remarkably well as a sacrificial template for our STG strategy, largely due to its intrinsic metastability. What truly captivated us, however, was the mechanism behind this transformation. We discovered that the STG process begins when the PA2PbI4 layer selectively dissolves in the alcohol-based solution, removing PA⁺ from the lattice. Simultaneously, the target PiEA2+ cation intercalates into the transient residual Pb-I framework, giving rise to a PbI2-(PiEA)I2 intermediate (Fig. 1). Density functional theory calculations revealed that this intermediate formation pathway is thermodynamically favourable – unlike the conventional route involving direct reaction between (PiEA)I2 and the perovskite surface, which is energetically unfavourable. This distinction explains why the STG strategy succeeds where other methods fail. The process ultimately enables efficient organic cation exchange between PA+ and PiEA2+, culminating in the in-situ formation of the desired chemically inert 2D interface, (PiEA)PbI4.
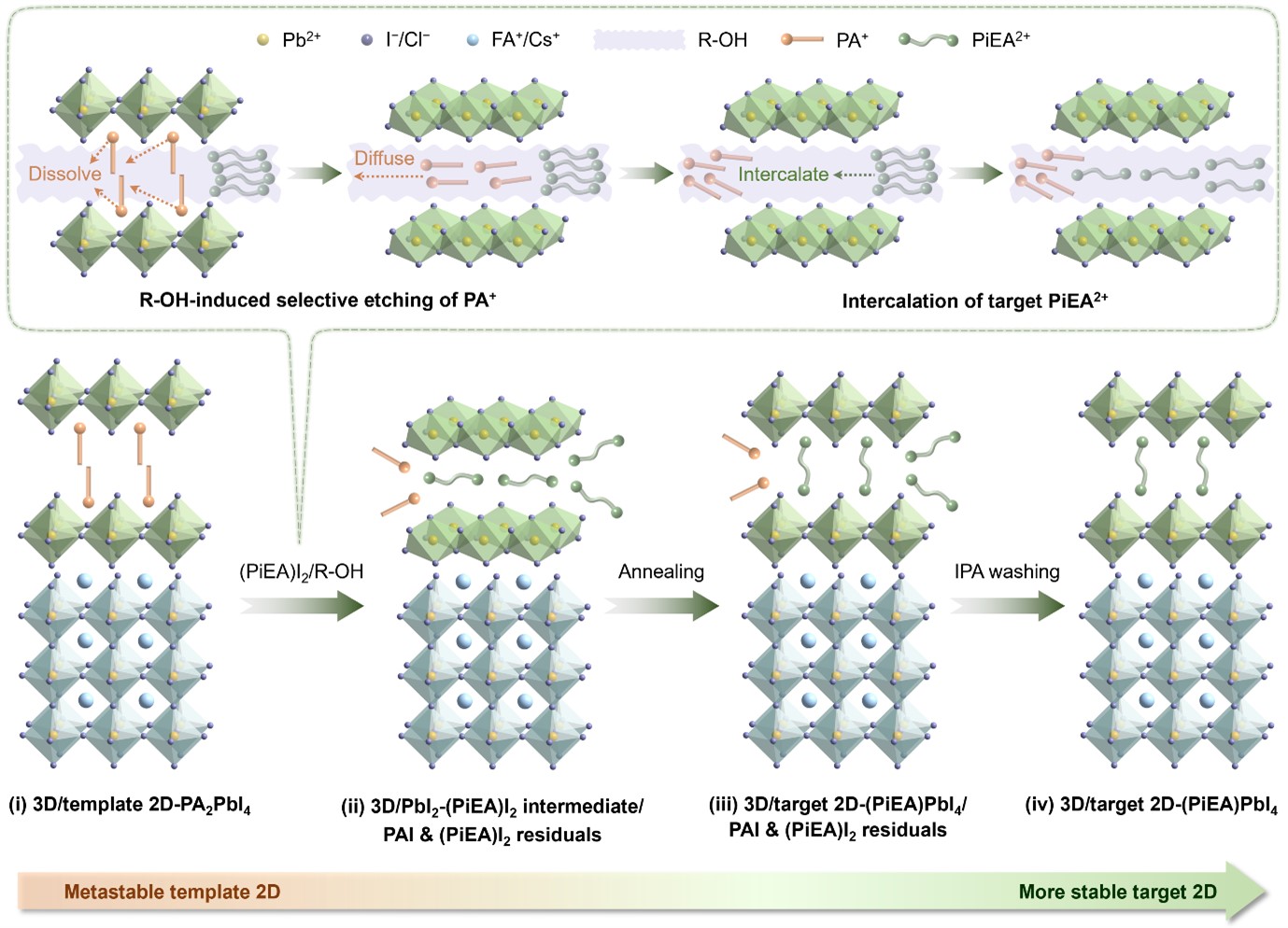
Fig. 1 | Potential mechanism underlying the STG strategy. Illustration of the underlying structural and compositional evolution governing the STG process for constructing the 3D/STG-target 2D-(PiEA)PbI4 architecture, which incorporates two key processes: R–OH-induced selective etching of the template’s bulky cation PA+ and concurrent intercalation of the target bulky cation PiEA2+.
What truly validated our effort was the remarkable performance of the resulting chemically inert 2D (PiEA)PbI4 interface. Most notably, it exhibited superior intrinsic chemical stability, offering substantially greater protection against environmental stresses than conventional 2D counterparts such as PEA2PbI4 (PEA = phenylethylammonium). Beyond acting as a robust barrier, the (PiEA)PbI4 interface also provided efficient chemical and field-effect passivation at the perovskite surface – effectively suppressing trap states and non-radiative recombination. These synergistic effects translated into impressive device performance: our 1-cm2 perovskite solar cells achieved a power conversion efficiency of 25.1%, ranking among the highest reported for this device size. In terms of stability, the results were equally compelling – the devices retained over 93% of their initial efficiency after 1,000 hours of continuous operation under maximum power point tracking, and 98% after 1,100 hours of thermal aging at 85 °C – highlighting the long-term reliability conferred by this newly accessible LD interface.
What excited us even further was the versatility and scalability of the STG strategy. While the chemically inert 2D (PiEA)PbI4 interface served as our proof of concept, the same strategy proved remarkably generalizable. It enabled the successful construction of a broad range of chemically inert LD interfaces – including other lead-based systems such as (3MTPA)2PbI4 (3MTPA = 3-(methylthio)propylammonium), (BPMA)2PbI4 (BPMA = biphenyl-4-yl-methylammonium), and (PrEA)PbI4 (PrEA = 2-pyrrolidin-1-ium-1-ylethylammonium), as well as the lead-free analogue (PiEA)SnI4. Beyond compositional flexibility, the STG process also demonstrated excellent compatibility with various fabrication methods. In addition to spin-coating, it could be readily extended to scalable deposition techniques such as blade-coating – marking a critical step toward large-area film fabrication and commercial viability.
Collectively, these findings establish STG as a versatile and practical interface engineering platform that unlocks a previously inaccessible class of chemically inert LD interfaces – opening a new design space for perovskite optoelectronics spanning photovoltaics, LEDs, lasers, and photodetectors.
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
What are SDG Topics?
An introduction to Sustainable Development Goals (SDGs) Topics and their role in highlighting sustainable development research.
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in