Metabolic profiling of galectin-1 and galectin-3: a cross-sectional, multi-omics, association study
Published in Cell & Molecular Biology, General & Internal Medicine, and Anatomy & Physiology
How does obesity lead to disease? The modern lifestyle with a high intake of calorie-dense foods, and sedentarism has resulted in a global increase in the prevalence of obesity. This is problematic, as individuals with obesity have an increased risk of developing insulin resistance, dyslipidemia and type 2 diabetes (1). Underlying mechanisms for this transition have been extensively studied, and drugs such as GLP-1 analogs are already available to facilitate weight loss and improve metabolic alterations. Nonetheless, the large amount of individuals affected by obesity and its complications, mean that we should to continue the search for additional mechanisms through which obesity can lead to metabolic diseases.
In recent years, the two proteins galectin-1 and galectin-3 have been identified as potential players in the transition of obesity to type 2 diabetes (2, 3). These galectins are part of a full family of proteins, which all share the ability to interact with carbohydrates in the body. Galectins are known regulators of tissue reorganization, and inflammation. This may also explain why these proteins may also be related to metabolic disease.
This is the rationality behind our current study. We wanted to explore if the metabolic effects of these galectins were readily identifiable in individuals from a general population. We also wanted to see if we could find any link to obesity, and examine if we could find potential mechanisms behind this link. Even more, as these proteins are also part of the same family, we were curious to see if there were any similarities and differences between the two proteins.
To complete these objectives, we measured galctin-1 and galectin-3 in the plasma of more than 500 individuals in the population-based Prospective investigation of Obesity, Energy and Metabolism (POEM) study on 50-year olds living in the city of Uppsala in Sweden. We also examined the participants with a range of different ‘omics methods, including imiomics (imaging-omics based on data from a whole body MRI), as well as proteomics and metabolomics of the blood. After these examinations, we tried to find any associations between the circulating levels of each galectin with these different examinations.
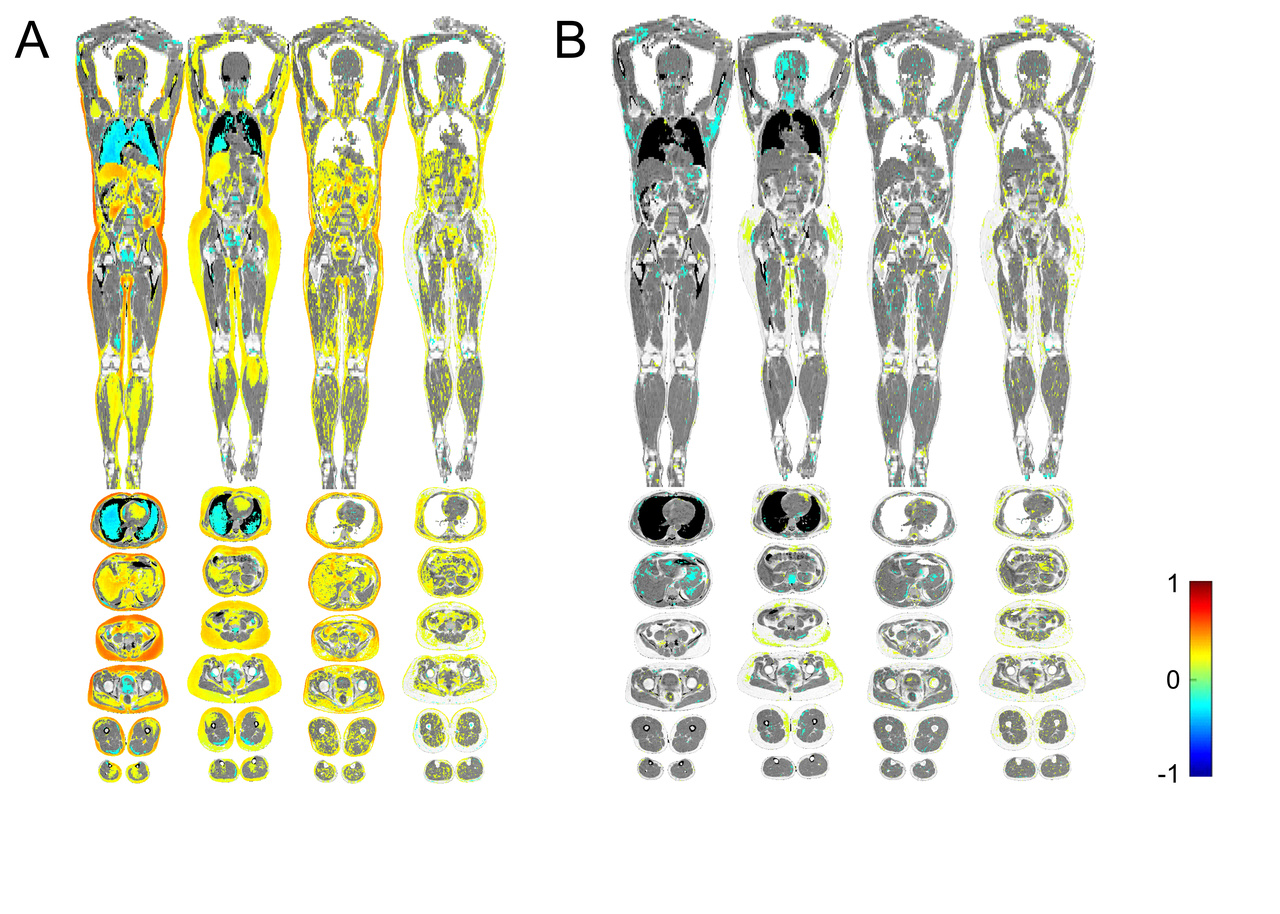
The results? The imiomics showed us that plasma galectin-1 levels clearly correlated with more body fat and more lipids stored in other tissues (A). In contrast, we did not find any correlation between galectin-3 and the amount of body fat in the imiomics (B). These results fit well with previous studies on galectin-1, which have found that high galectin-1 levels correlate with larger fat cells, and with BMI (2, 4). The neutral results on galectin-3 in adipose tissue aligns with the idea that galectin-3 involved in human metabolism originates from hematopoietic cells (3).
So what did we find when associating the galectins with different measurements in human metabolism? Well, increased galectin-1 levels were associated with higher C-peptide levels, and increased insulin resistance, and galectin-3 levels with higher levels of fasting insulin. Both galectins were also associated with lower levels of fasting glucose. Furthermore, the associations between the two galectins and different fatty acids were virtually identical. These observations indicate that both galectins may play a role in human metabolism, and also metabolic diseases related to insulinresistance. Our observations are interesting, as they indicate effects in human, which have previously been demonstrated in mice. Studies in animal models have found that galectin-1 knockout mice on high fat diet gain less weight than normal mice, and that galectin-1 interacts with the activity of the adipose tissue transcription factor PPAR-γ (5). Previous studies on galectin-3 have also indicated interactions with PPAR-γ as well as the lipid uptake marker CD36 (6).
To understand the potential regulatory pathways behind our observed metabolic associations, we also explored how the galectins related with the circulating proteome. As might be expected, given the associations with all types of lipids, we found that both galectins associated with the proteins fatty acid binding protein (FABP) 4 and -5, important in lipid metabolism. Higher galectin-1 levels were also associated with higher levels of FABP1, perilipin-1, and perilipin-3, which are other proteins involved in lipid metabolism. Galectin-1 was also associated with higher levels of angiopoietin-like 4, leptin, and transforming growth factor (TGF)-β1, which are proteins involved in transcriptional regulation of the white adipocyte differentiation pathway. We were intrigued by the consistency between our proteomic analysis and the imiomics examination, which both suggested that galectin-1, but not galectin-3, is associated with adipose tissue morphology and maturation.
Our exploration of proteins associated with galectin-1 and galectin-3 revealed another similarity between the galectins when we explored the underlying pathways. This analysis indicated that IL-10 and TNF signaling pathways were associated with both galectins in this general population. These pathways are known to be involved in metabolic inflammation, and could provide an interesting link between previously known regulatory effects of these galectins in inflammation, and our observed metabolic associations with insulin and lipid metabolites.
To conclude, we did see that galectin-1 but not galectin-3 associated with the adipose tissue morphology and adipogenesis. But both galectins were associated with markers of insulin resistance, and the associations with triglycerides and different fatty acids were virtually identical. While this study is only of observational nature, we will continue to further explore the potential metabolic effects of galectins for their roles in human lipid metabolism, and diabetes in experimental studies. The similarities in association patterns also suggests that future studies should consider possible functional overlaps between different galectins.
- Fryk E, Olausson J, Mossberg K, Strindberg L, Schmelz M, Brogren H, et al. Hyperinsulinemia and insulin resistance in the obese may develop as part of a homeostatic response to elevated free fatty acids: A mechanistic case-control and a population-based cohort study. EBioMedicine. 2021;65:103264.
- Fryk E, Sundelin JP, Strindberg L, Pereira MJ, Federici M, Marx N, et al. Microdialysis and proteomics of subcutaneous interstitial fluid reveals increased galectin-1 in type 2 diabetes patients. Metabolism: clinical and experimental. 2016;65(7):998-1006.
- Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell. 2016;167(4):973-84 e12.
- Drake I, Fryk E, Strindberg L, Lundqvist A, Rosengren AH, Groop L, et al. The role of circulating galectin-1 in type 2 diabetes and chronic kidney disease: evidence from cross-sectional, longitudinal and Mendelian randomisation analyses. Diabetologia. 2022;65(1):128-39.
- Baek JH, Kim DH, Lee J, Kim SJ, Chun KH. Galectin-1 accelerates high-fat diet-induced obesity by activation of peroxisome proliferator-activated receptor gamma (PPARgamma) in mice. Cell Death Dis. 2021;12(1):66.
- Yu H, Yang F, Zhong W, Jiang X, Zhang F, Ji X, et al. Secretory Galectin-3 promotes hepatic steatosis via regulation of the PPARgamma/CD36 signaling pathway. Cell Signal. 2021;84:110043.
Follow the Topic
-
International Journal of Obesity

This is a multi-disciplinary forum for research describing basic, clinical and applied studies in biochemistry, physiology, genetics and nutrition, molecular, metabolic, psychological and epidemiological aspects of obesity and related disorders.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in