Metallic WO2-Promoted CoWO4/WO2 Heterojunction with Intercalation-Mediated Catalysis for Lithium–Sulfur Batteries
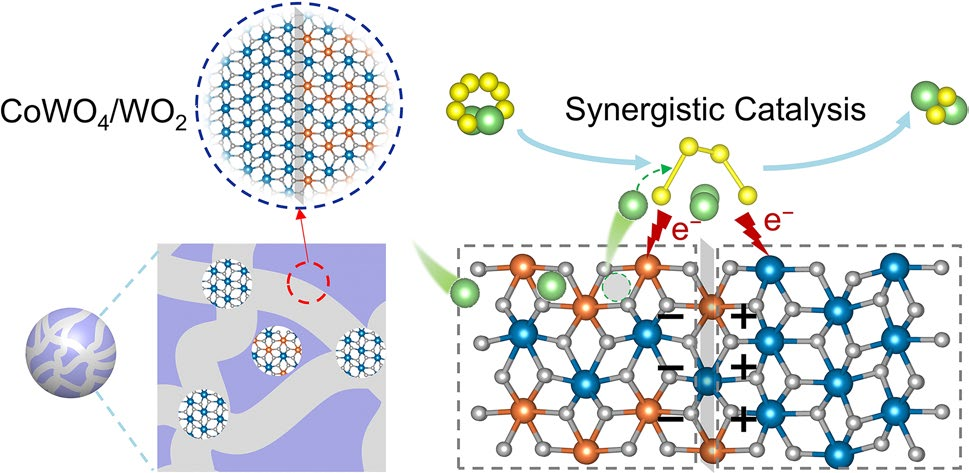
A research team led by Professors Xiaoyan Zheng, Huigang Zhang, and Tao Yang has reported a significant advance in Nano-Micro Letters on catalyst design for lithium–sulfur (Li–S) batteries. Their work introduces a rationally engineered CoWO4/WO2 heterojunction that leverages intercalation-mediated catalysis and metallic conductivity to simultaneously accelerate polysulfide conversion and suppress the shuttle effect—two major challenges hindering practical Li–S batteries.
Why Heterojunction Catalysts Matter
- Polysulfide Management: Li–S batteries suffer from the dissolution and migration of lithium polysulfides (LiPSs), which cause active material loss and poor cycling stability.
- Catalytic Enhancement: Traditional catalysts often balance strong adsorption with limited conductivity or vice versa, leaving performance constrained.
- Transport Dynamics: Efficient ion/electron pathways are critical for maintaining high sulfur utilization, especially under high rates and loadings.
Design Strategy of CoWO4/WO2
- Strong Adsorption: CoWO4 provides robust chemisorption of LiPSs and weakens S–S bonds, lowering reaction barriers.
- Metallic Conduction: The in situ–formed WO2 phase introduces metallic electron highways and donates electrons to CoWO4, enhancing catalytic activity.
- Li-Ion Intercalation: Directional intercalation channels in CoWO4 act as Li-ion reservoirs, enabling fast ion diffusion and continuous transport.
- Synergistic Interfaces: The heterointerface promotes charge redistribution, orbital interactions, and efficient Li–S bond activation.
Performance Highlights
- High Capacity: The heterostructure achieves 1262 mAh g-1 at 0.1 C, outperforming single-component counterparts.
- Rate Capability: Even at high current densities, the electrode maintains stable dual-plateau discharge profiles with low polarization.
- Cycling Durability: At 1 mg cm-2 sulfur loading, the electrode shows a minimal decay rate of 0.038% per cycle over 1000 cycles. Under high loading (5 mg cm-2), it retains 79.1% capacity after 235 cycles.
- Shuttle Suppression: In situ Raman and XRD confirm efficient polysulfide conversion with negligible shuttle effect.
Future Outlook
This study demonstrates how integrating adsorption, catalysis, and ion transport within a single heterojunction architecture can redefine Li–S battery design. The intercalation-mediated mechanism offers a blueprint for next-generation multifunctional catalysts, potentially enabling scalable, high-energy, and long-life Li–S systems. Beyond CoWO4/WO2, the approach provides a paradigm for constructing heterostructures that combine metallic promoters with ion-intercalating hosts, advancing practical energy storage solutions.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in