
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)is an emerging coronavirus that has caused globally over 30 million laboratory-confirmed cases of coronavirus disease 2019(COVID-19)including more than 1 million death since December 2019. This pandemic poses an unprecedented challenge to rapidly identify effective drugs for the prevention and treatment of this disease. Since there has still been no effective vaccine against SARS-CoV-2, the discovery and development of virus-specific antiviral agents by repurposing currently available drugs represents the most practical route for the rapid identification of anti-COVID-19 agent. Remdesivir, a broad-spectrum antiviral drug, has been reported to show efficacy against SARS-CoV-2. However, the drug’s global shortage, relatively high price and lack of significant clinical benefits in patients with severe COVID-19 have so far limited its widespread use. Other ongoing clinical trials on a series of antiviral agents are demonstrating the big challenge to improve the clinical outcomes of patients. Therefore, more intense efforts are urgently needed to evaluate a wider spectrum of clinically approved drugs by alternative strategies.
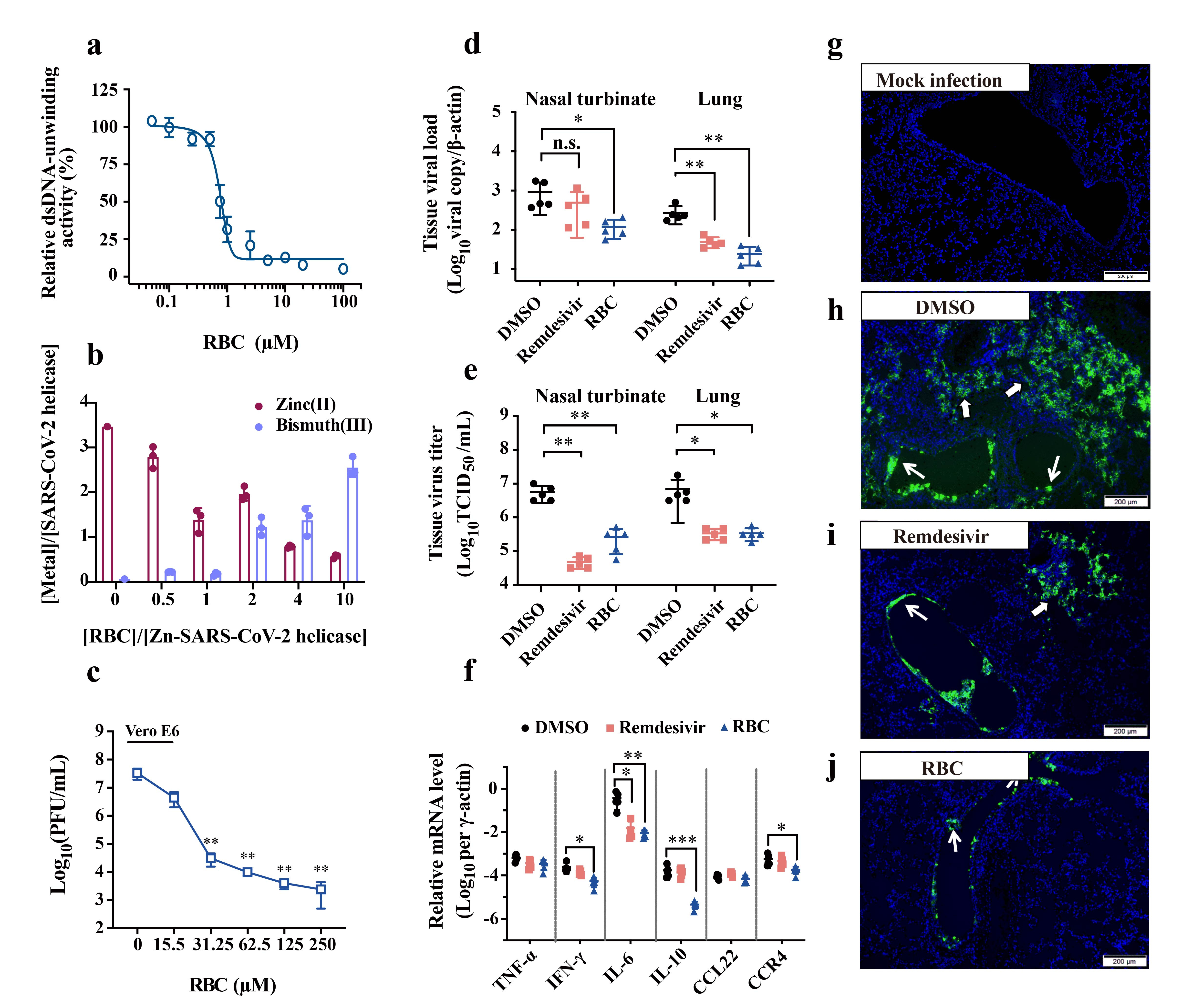
Accumulative studies demonstrate that SARS-CoV-2 produces a battery of viral enzymes and proteins that are essential for viral replication cycles, including structural proteins and nonstructural proteins (Nsp). Intervention at viral entry or replication stage allows those therapeutics to be effective. Significantly, several Nsps that are validated to be responsible for some crucial biological functions during the replication, including papain-like protease (Nsp3), RNA-dependent RNA polymerase (Nsp12), helicase (Nsp13), proofreading exoribonuclease (Nsp14), and 2'-O-ribose methyltransferase (Nsp16). Our studies began after we excavated Zn(II) ions play crucial role in all of the above-mentioned Nsps. Intriguingly, we validated in previous studies that some inorganic pharmaceutics, e.g., Bi(III) drugs, defunctionalized key zinc-enzyme in bacteria via a distinct Bi(III)-to-Zn(II) displacement mechanism, inferring that those Nsps may serve as promising targets of potential therapeutic drugs. Thus, our strategy to tackle this issue was to choose some available metallo-drugs that are capable of inhibiting viral replication by disrupting biological function of Nsps in SARS-CoV-2. In our most recent studies, we identify ranitidine bismuth citrate (RBC), a commonly used anti-ulcer drug for the treatment of Helicobacter pylori-associated infection, through a primary screening over a set of metallodrugs and related compounds, as a potent anti-SARS-CoV-2 agent, both in vitro and in vivo. RBC exhibited low cytotoxicity and protected SARS-CoV-2-infected cells with a high selectivity index of 975. Importantly, RBC suppressed SARS-CoV-2 replication, with decreased viral loads in both upper and lower respiratory tracts, and relieved virus-associated pneumonia in a golden Syrian hamster model. In vitro studies showed that RBC and its related compounds exhibited inhibition towards both the ATPase (IC50=0.69 µM) and DNA-unwinding (IC50=0.70 µM) activities of the SARS-CoV-2 helicase via a metal displacement route by irreversible displacing crucial zinc(II) ions from the zinc-finger domain of the enzyme with bismuth(III) ions.
Altogether, the presented data provide a proof-of-principle concept highlighting the viral helicase as a druggable target for SARS-CoV-2 and the clinical potential of Bi(III) drugs or other related metallodrugs for the treatment of SARS-CoV-2 infection.
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in