Microfluidics for bottom up studies of biofilms and bubbles
Published in Microbiology

On the nature of “skeletal” biofilm patterns, “hidden” heterogeneity and the role of bubbles to reveal them
Jesse Greener, nph Biofilms and Microbiomes, 2019, 5, 12
It was the hunt for a good analytical challenge that brought us to study biofilms, but what kept our attention was the amazing potential for impact in medicine and technical applications like bio-catalysis, bioenergy and even “living sensors”. The common thread for our group has always been microfluidics. Behind the buzz, microfluidics is a wonderful approach to study living cells and their communities. Extremely accurate perturbations coupled with in situ monitoring gives gratifying responses in near real-time.[1] Our group only started vigorously investigating biofilms 7 years ago. Initial efforts were passive; we watched and analysed time-lapse images of growth after inoculation and were rewarded with atypical observations on a range of physiochemical phenomena, like an unreported method of streamer formation by “sudden partial detachment”[2] and a rapid transition to highly viscous state in a matter of hours.[3] In this approach, even problems like surface adhered bubbles, a common plague for microfluidics research, could be viewed in a new light.[4] Clicking on Figure 1 presents a dramatic time-lapse confocal image sequence at the (glass) attachment surface during the transformation of a thin confluent Pseudomonas sp. bacterial layer into mushroom structures. With this last figure in mind, we were excited to read about other microfluidic biofilm experiments like the one from Jang et al. where they produced similar patterns using the passage of a slow moving bubble across nascent biofilms.[5] They proposed that the patterns which were revealed were actually predetermined by the early accumulation of extracellular polymeric molecules, the non-living portion of a biofilm that protects and stabilizes the bacteria within. We believe these early formations would define the future structure of the surface attachment points in a mature, such as is seen at the end of the time-lapse video below.
In the next step, we are conducting surface sensitive spectroscopy at the biofilm attachment surface to learn more about the surface chemistry during the development of the skeletal attachment in the presence of surface adhered bubbles. We hope that this line of inquiry can provide hints about methods to control or disrupt formation of nascent biofilms.
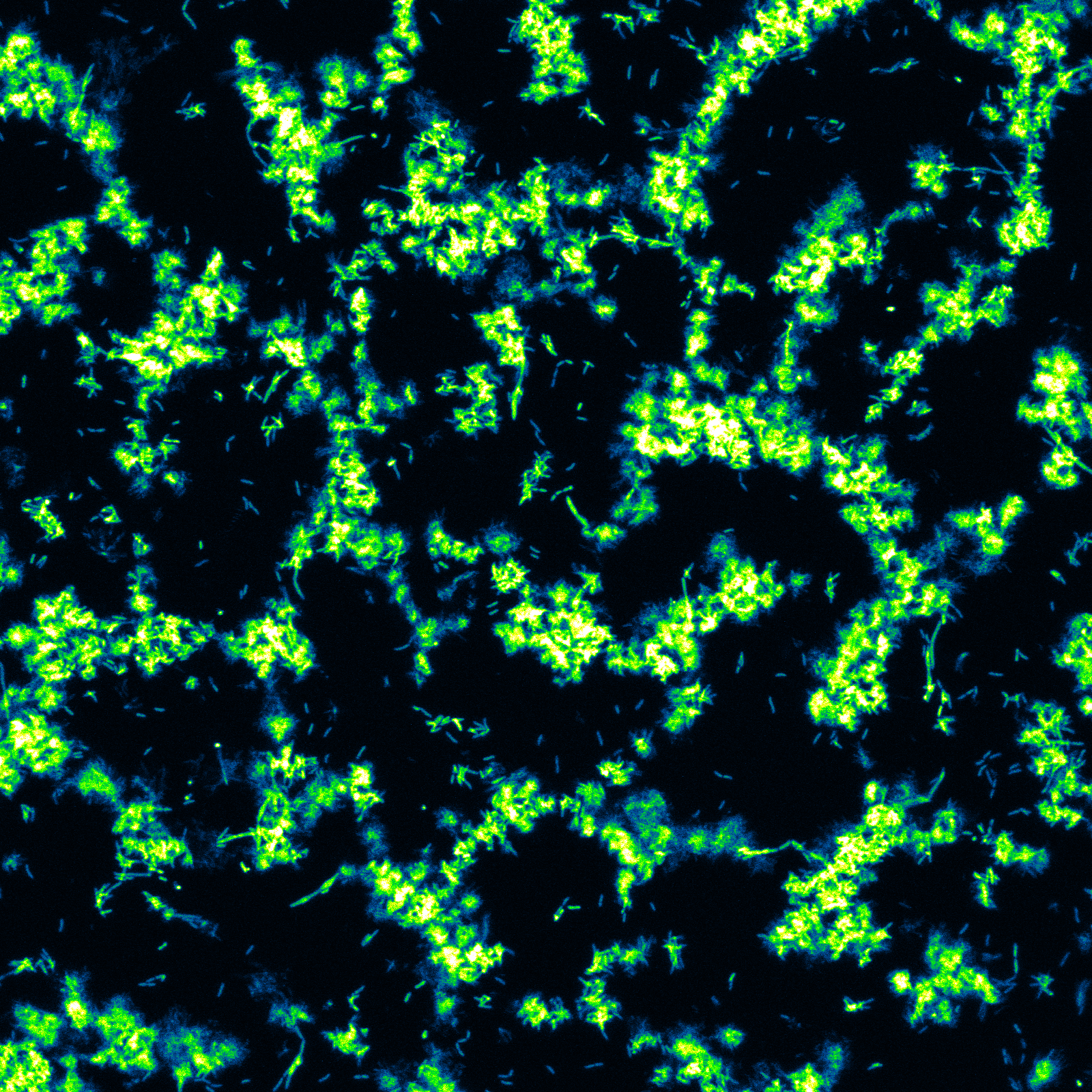
Figure 1. A false coloured still from a time-lapse image sequence at the biointerface using confocal laser scanning microscopy. Click to see the full time-lapse image sequence of a nascent biofilm as it transforms to skeletal structure.
[1] M. Pousti, M.P. Zarabadi, M. A. Amirdehi, F. Paquet-Mercier and J. Greener, "Microfluidic bioanalytical flow cells for biofilm studies: A review", Analyst, 144, 68-86 (2019).
[2] M. Parvinzadeh Gashti, J. Bellavance, O. Kroukamp, G. Wolfaardt, S. M. Taghavi, J. Greener, "Live-streaming: Time-lapse video evidence of novel streamer formation mechanism and varying viscosity", Biomicrofluidics, 9, 041101 (2015).
[3] F. Paquet-Mercier, M. Parvinzadeh Gashti, J Bellevance, S. Mohammad Taghavi, J. Greener, "Through thick and thin: a microfluidic approach for continuous measurements of biofilm viscosity and the effect of ionic strength", Lab Chip, 16, 4710-4717 (2016).
[4] Farnaz Asayesh, Mir Pouyan Zarabadi and Jesse Greener, "A new look at bubbles during biofilm inoculation reveals pronounced effects on growth and patterning", Biomicrofluidics, 11, 064109 (2017).
[5] H. Jang, R. Rusconi and R. Stocker, "Biofilm disruption by an air bubble reveals heterogeneous age-dependent detachment patterns dictated by initial extracellular matrix distribution", NPJ Biofilms Microbiomes, 3, 1-7 (2017).
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: Feb 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in