More than a than a battle of males: the association between alpha male takeovers, social networks, and gut microbiota composition
Published in Ecology & Evolution
When I returned to Boabeng-Fiema in Ghana for another data collection season, there was complete chaos in one of the social groups of black-and-white colobus monkeys. There were three new adult males in WW group who were chasing and contact fighting with the old alpha male and with each other (Fig. 1). During the first two months, a newborn infant and three one-year old immatures were attacked by males but survived the minor wounds they sustained (Fig. 2), and two of the new males died after getting wounded or falling out of a tree. The surviving new male kept fighting with the old alpha male for a year with frequent changes in alpha status between the two. Four infants were born 6-9 months into the data collection season, but only one survived (Fig. 2). The other three infants died when they were approximately one month old.

Fig. 1. The old alpha male is surrounded by immatures (top) shortly before one of the new immigrant males approaches, the immatures scatter, and a fight breaks out between the males (bottom) in WW group.
We refer to this type of alpha male replacement process as a slow alpha male takeover. Slow takeovers take several months and often occur when several new immigrate males try to oust the old alpha male and also fight amongst themselves until only one male remains (Sicotte et al., 2017). The prolonged elevated levels of male aggression are disruptive to all group members. During slow takeovers with prolonged social turmoil, females are more likely to disperse from the group (Sicotte et al., 2017). Alpha male takeovers are also associated with high rates of male attacks on unweaned infants (Fig. 2), and infanticide accounts for almost 40% of infant mortality in this study population (Teichroeb & Sicotte, 2008).

Fig. 2. Wendy with infant Willow before and after Willow was wounded (top left and top middle), infant Chai after getting wounded by a male (top right), and Marla with infant Mad Max who was born during the study period but never seen to be attacked by males (bottom).
This study (Samartino et al., 2024) focused on a less well-investigated topic, namely how alpha challenges and takeovers are linked to female social network and gut microbiota dynamics. Gut microbiota composition in many group-living animals is tightly linked to social group identity and social interactions (Archie & Tung, 2015), and social transmission of microbes may stabilize an individual’s gut microbiota (Sarkar et al., 2020). However, the group’s social network may change during alpha male takeovers as some group members may seek out the new males while others, like females with infants vulnerable to infanticide, may avoid them.
To investigate social network dynamics, we compared adult female long-term (12-month) and short-term (3-month) 1m proximity networks. In two social groups that did not experience alpha male takeovers, the long-term and short-term social networks were closely correlated, indicating that social networks were stable over time. In each of these two socially stable groups, the long-term proximity network predicted gut microbiota similarity among females.
In WW group with the alpha male takeover, the long-term and short-term proximity networks were not correlated, which indicated that their social network changed over time (Fig. 3). Likely because the long-term network did not indicate how often females were in proximity during the time of gut microbiota sampling, the long-term network did not predict female gut microbiota similarity in this group. Female gut microbiota similarity was correlated with the short-term proximity network, and gut microbiota similarity increased with time spent in proximity during the 3-month period preceding and overlapping the gut microbiota sampling period.
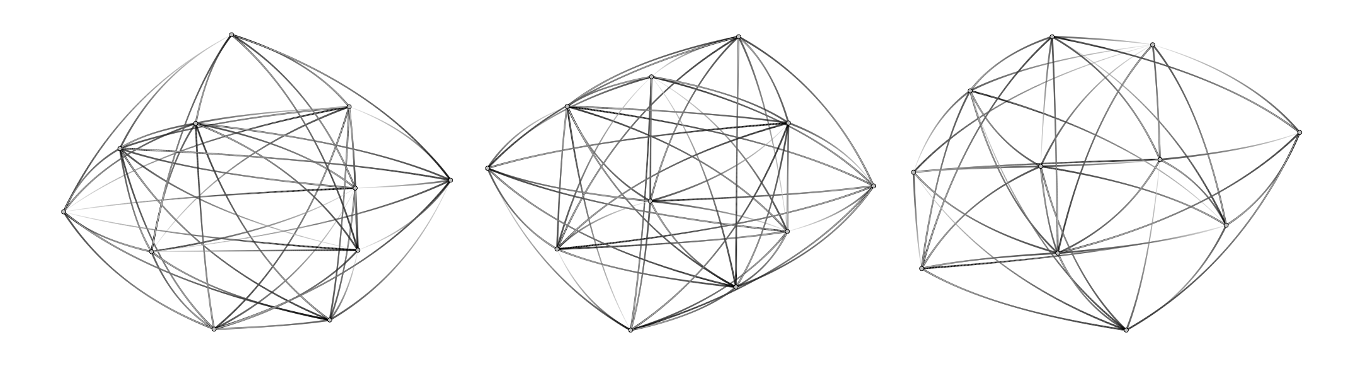
Fig. 3. WW group directed unweighted 1m approach network before, during, and after gut microbiota sample collection.
In WW group from which several infants disappeared during the alpha male takeover, female gut microbiome composition was also predicted by whether the females experienced infant loss (Fig. 4). Females whose infants died shortly before or after gut microbiota sampling had more similar gut microbiota to each other than to the other females in the group.
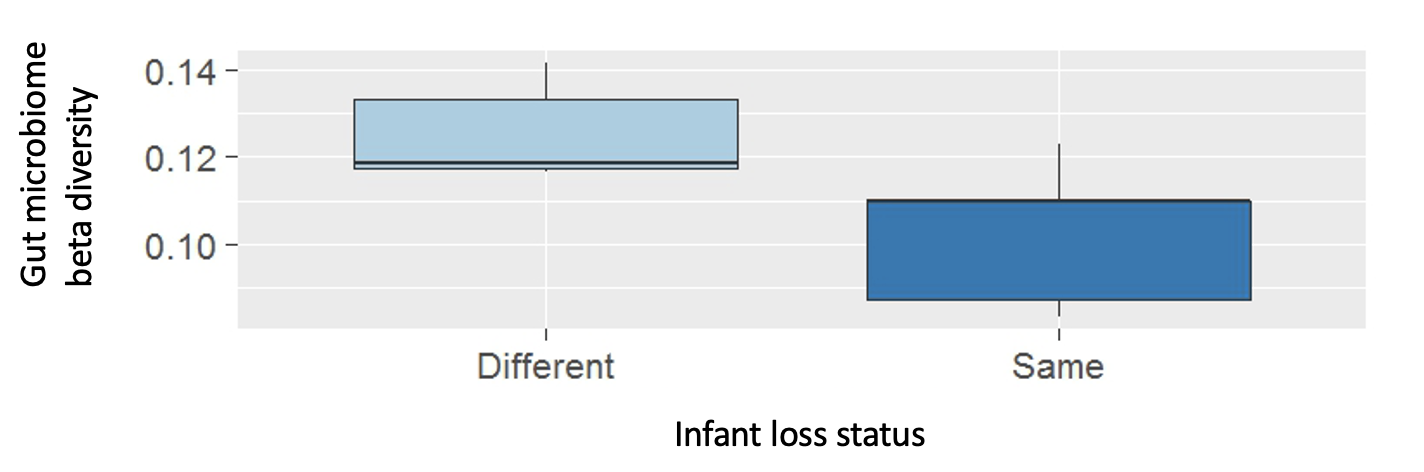
Fig. 4. Gut microbiome dissimilarity for female dyads with different infant loss status (i.e., one female lost an infant while the other one did not) and same infant loss status (i.e., both or none of the two females lost infants).
Finally, we also investigated whether alpha male instability was associated with adult female gut microbiota composition in 53 samples collected from adult females in 6 social groups. A small percentage of the variation in gut microbiota composition were explained by whether the female resided in a group with alpha male instability.
Many studies highlight the importance of social transmission of microbes in group-living animals (Archie & Tung, 2015), and social microbe transmission can explain why social network dynamics associated with alpha male takeovers predicted gut microbiome composition in this study. In addition, experiencing alpha male challenges and takeovers are likely stressful experiences. When a group’s alpha male has been wounded or group members have died, individuals show elevated glucocorticoid levels (King et al., 2023), which in turn are associated with variation in gut microbiota composition (Hickmott et al., 2022; Stothart et al., 2016). Thus, the combination of changing social networks and exposure to stressors may explain gut microbiota dynamics during alpha male takeovers. Because group members experience prolonged exposure to stressors and social instability during slow male takeovers, it will be important to further investigate its long-term consequences.
References
Archie, E. A., & Tung, J. (2015). Social behavior and the microbiome. Current Opinion in Behavioral Sciences, 6, 28–34. https://doi.org/10.1016/j.cobeha.2015.07.008
Hickmott, A. J., Boose, K. J., Wakefield, M. L., Brand, C. M., Snodgrass, J. J., Ting, N., & White, F. J. (2022). A comparison of faecal glucocorticoid metabolite concentration and gut microbiota diversity in bonobos (Pan paniscus). Microbiology (Reading, England), 168(8). https://doi.org/10.1099/mic.0.001226
King, A. G., Edwards, P. D., Cote, S., Palme, R., Boonstra, R., & Sicotte, P. (2023). Assessing stress in wild black-and-white colobus monkeys non-invasively. General and Comparative Endocrinology, 334, 114212. https://doi.org/10.1016/j.ygcen.2023.114212
Samartino, S., Christie, D., Penna, A., Sicotte, P., Ting, N., & Wikberg, E. C. (2024). Social network dynamics and gut microbiota composition during alpha male challenges in Colobus vellerosus. Primates, 65, 299–309.
Sarkar, A., Harty, S., Johnson, K. V.-A., Moeller, A. H., Archie, E. A., Schell, L. D., Carmody, R. N., Clutton-Brock, T. H., Dunbar, R. I. M., & Burnet, P. W. J. (2020). Microbial transmission in animal social networks and the social microbiome. Nature Ecology & Evolution, 4(8), 1020–1035. https://doi.org/10.1038/s41559-020-1220-8
Sicotte, P., Teichroeb, J. A., Vayro, J. V., Fox, S. A., Bădescu, I., & Wikberg, E. C. (2017). The influence of male takeovers on female dispersal in Colobus vellerosus: Takeovers and female dispersal in colobus. American Journal of Primatology, 79(7), e22436. https://doi.org/10.1002/ajp.22436
Stothart, M. R., Bobbie, C. B., Schulte-Hostedde, A. I., Boonstra, R., Palme, R., Mykytczuk, N. C. S., & Newman, A. E. M. (2016). Stress and the microbiome: Linking glucocorticoids to bacterial community dynamics in wild red squirrels. Biology Letters, 12(1), 20150875. https://doi.org/10.1098/rsbl.2015.0875
Teichroeb, J. A., & Sicotte, P. (2008). Infanticide in ursine colobus monkeys (Colobus vellerosus) in Ghana: New cases and a test of the existing hypotheses. Behaviour, 145(6), 727–755. https://doi.org/10.1163/156853908783929160
Follow the Topic
-
Primates

This is an international journal of primatology whose aim is to provide a forum for the elucidation of all aspects of primates.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in