Nationwide increases in anti-SARS-CoV-2 IgG antibodies between October 2020 and March 2021 in the unvaccinated Czech population
Published in Healthcare & Nursing
To address the lack of regional data and to investigate the dynamics of seroprevalence of anti-SARS-CoV-2 IgG antibodies in the region, a nationwide prospective seroconversion study (PROSECO) was initiated in the Czech Republic. The PROSECO study provide key data from the heavily affected Central European region and contribute to the epidemiological and serological characteristics of the SARS-CoV-2 infection.
The aim was to study the dynamics of seroconversion in three six-month-long periods: 1) the second epidemic wave (unvaccinated population); 2) the mass vaccination period; and 3) the post-vaccination period with seasonal viral challenge.
Similar to most European countries, the first COVID-19 epidemic wave in the Czech Republic in spring 2020 produced a relatively low incidence. At the peaks of the second wave, however, over 100 confirmed cases per 100,000 persons were diagnosed daily and the Czech Republic ranked among the countries with the greatest burden of COVID-19 in Europe and in the world.
All 30,054 participants of the PROSECO study were recruited between October 2020 and March 2021, thus covering all three epidemic peaks (November 2020, January and March 2021) of the second COVID-19 epidemic wave (Figure). This allowed to follow dynamics of seroconversion of anti-SARS-CoV-2 IgG antibodies in the immunologically naive and unvaccinated population.
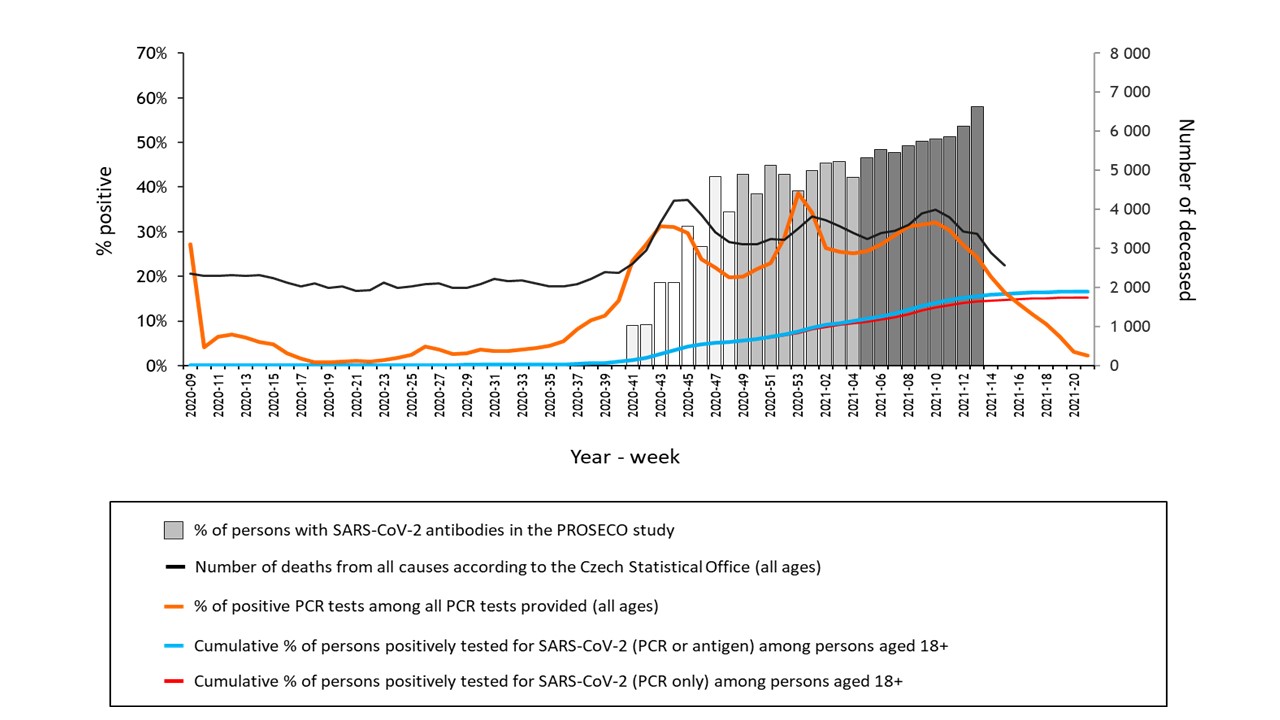
Figure Weekly seroprevalence of SARS-CoV-2 antibodies in pandemic wave before vaccination in the Czech Republic. Dynamics of the COVID-19 pandemic in the Czech Republic and seroprevalence in the first phase of the PROSECO study between October 2020 and March 2021.
To the best of our knowledge, the PROSECO study is the largest study on seroprevalence of anti-SARS-CoV-2 IgG antibodies in Central and Eastern Europe. Major strengths of the study are its size, coverage, start before vaccination period and on-going longitudinal follow-up. In contrast to other larger studies, the levels of antibodies were assessed in the harmonized network of accredited clinical laboratories.
Data from the first phase indicate that a percentage of seropositive individuals may be substantially higher than estimates based on governmental data on cumulative viral positivity prevalence as at least one third of seropositive participants were asymptomatic, and 28% of participants who developed antibodies against SARS-CoV-2 never underwent PCR testing. The study participants will be re-assessed in the second (April 2021-September 2021) and third (October 2021-March 2022) PROSECO phases to further study the post-infection/post-vaccination dynamics of seroconversion in/after a period of massive vaccination.
Regional seroprevalence data are instrumental for successful management of the next phases of global pandemic. Case definitions enable public health officials to classify and count cases consistently across reporting jurisdictions. The results of the PROSECO study imply the addition of seropositivity to the laboratory criteria for case definition such as seropositivity of SARS-CoV-2 specific antibodies in unvaccinated persons or antibody positivity against SARS-CoV-2 antigens in vaccinated persons not contained or encoded in the vaccine.
Our data confirm the rapidly increasing prevalence in the Czech population during the rising pandemic wave prior to the beginning of vaccination. The planned second and third assessment of the study participants (April 2021 – September 2021, October 2021 – March 2022) will provide valuable evidence on the seroprevalence changes following vaccination and persistence of antibodies resulting from natural infection and vaccination.
https://www.nature.com/articles/s43856-022-00080-0.pdf
References
Long, Q.-X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine 26, 845-848, doi:10.1038/s41591-020-0897-1 (2020).
Caturegli, G., Materi, J., Howard, B. M. & Caturegli, P. Clinical Validity of Serum Antibodies to SARS-CoV-2 : A Case-Control Study. Ann Intern Med 173, 614-622, doi:10.7326/m20-2889 (2020).
Sakurai, A. et al. Natural History of Asymptomatic SARS-CoV-2 Infection. New England Journal of Medicine 383, 885-886, doi:10.1056/NEJMc2013020 (2020).
Payne, D. C. et al. SARS-CoV-2 Infections and Serologic Responses from a Sample of U.S. Navy Service Members - USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep 69, 714-721, doi:10.15585/mmwr.mm6923e4 (2020).
Stringhini, S. et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 396, 313-319, doi:10.1016/s0140-6736(20)31304-0 (2020).
Rostami, A. et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect 27, 331-340, doi:10.1016/j.cmi.2020.10.020 (2021).
Reicher, S. et al. Nationwide seroprevalence of antibodies against SARS-CoV-2 in Israel. Eur J Epidemiol, 1-8, doi:10.1007/s10654-021-00749-1 (2021).
Russell, M. W., Moldoveanu, Z., Ogra, P. L. & Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front Immunol 11, 611337, doi:10.3389/fimmu.2020.611337 (2020).
WHO COVID-19 Case definition. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 [05.02.2022]
Case definition for coronavirus disease 2019 (COVID-19), as of 3 December 2020. Available at: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition [05.02.2022]
SARS-CoV-2 Serology for COVID 19 certificate. Available at: https://swissmicrobiology.ch/en/cccm-recommendation-for-sars-cov-2-serology-for-covid19-certificate [05.02.2022]
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in