Opening a treasure trove: unlocking saponin biosynthesis in soapwort
Published in Plant Science

Plant specialized metabolites:
Plants are incredible chemists. As sessile organisms, they have evolved to produce an amazing array of compounds to protect themselves against harsh abiotic and biotic stresses such as UV radiation, pathogens and herbivores. These compounds are collectively known as plant natural products or specialized metabolites. As they offer a wide range of bioactivities, humans, since time immemorial, have relied on plants as sources of medicine, flavours, perfumes, dyes and many more. Even today, we still rely on plants for these uses. If you had a cup of coffee today to keep you going, you have already consumed a plant natural product, caffeine from beans of the coffee plant (Coffea spp.). Maybe you are a tea person and enjoyed a nice aromatic cup of tea made from the tea plant (Camellia sinensis) instead. Perhaps you had a headache and have taken some painkillers, like aspirin, which is originally driven from the willow tree (Salix spp.). One way or another, we consume and use plant natural products in our everyday lives.
Soapy saponins:
There are many more uses of plant natural products. For example, did you know that plants are also a source of soap? Many plants produce a type of specialized metabolites known as saponins. These have soap-like properties due to their amphiphilic nature, composed of a hydrophobic triterpene core which is decorated by hydrophilic sugar chain(s). In plants, saponins are generally believed to have defensive roles, providing protection against fungi, microbes and molluscs1. Humans have used plant extracts containing high amounts of saponins as soap and detergent, as well as in folk medicine for many centuries. Saponins continue to gain interests as agents of novel therapeutics, for example, as vaccine adjuvants. Most notably, QS-21, a complex triterpene saponin isolated from the Chilean soapbark tree (Quillaja saponaria), is a valuable immunostimulatory adjuvant used in human vaccines against shingles, malaria and COVID-192,3.
Soapwort - not just a pretty flower:
Saponaria officinalis, commonly known as soapwort, is a perennial flowering plant in the Caryophyllaceae family that is native to Eurasia. You may have seen them growing around riverbanks, meadows, roadsides, but really anywhere as they are quite hardy. Personally, I think they make excellent gardening flowers as they grow well in most conditions and hardly need much tending, yet they award you with pretty, white to pinkish flowers with delicate sweet scents (perhaps you already have them in your garden as you are an owner of impeccable taste).

Beyond their ornamental uses, they also have functional uses with deep-rooted roles in human history. The name saponaria is derived from the Latin word sapo or saponis, which means soap, and the word officinalis is a Latin word for herbal medicine. And yes, you guessed it! Soapwort extracts have been traditionally used as soap and medicine. Even today, soapwort extracts are still used in cosmetic, nutraceutical and phytomedicinal products. You can even opt to make your own natural, gentle soap using soapwort, which I will not reveal here but straight-forward instructions can be easily found on the internet.

Soapwort, saponins, saponariosides:
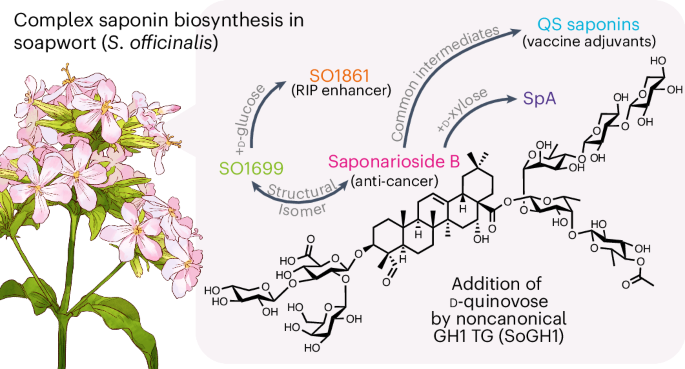
The well-known detergent properties of soapwort are due to the large amounts of saponins in the plant extracts. Currently, most applications of soapwort rely on plant extracts rather than individual saponin species. However, biochemical investigations of pure soapwort saponins may reveal their new pharmaceutical potentials. So how can we obtain good amounts of pure compounds to do this? Well, this is where it gets a little tricky. Soapwort extracts contain complex mixtures of similar saponins, which makes isolation and purification of individual components very time consuming and challenging. To exacerbate the situation, as these compounds are highly decorated (often acylated with 6-8 sugars), chemical synthesis approaches would be formidable. This is where we came in. We decided to investigate how soapwort makes these complex compounds. By uncovering the genes and enzymes involved in soapwort saponin biosynthesis, we can potentially engineer the original pathway in an alternative host system. But this approach came with its own challenges…
When we first started this project, nothing was known about saponin biosynthesis in soapwort. As soapwort produces many saponin species, we first had to establish our target saponin to uncover their biosynthetic pathway. The major saponins found in soapwort extracts are reported to be saponariosides A and B (SpA and SpB)4. Both are composed of a quillaic acid (oleanane-based triterpenoid) aglycone with sugar chains at the C-3 and C-28 positions. The only difference between the two saponins is the additional D-xylose unit in SpA. Remarkably, SpA and SpB show close structural similarities to the aforementioned QS-21 from soapbark, despite the two plants being only distantly related (Caryophyllales vs Fabales). Thus potentially, SpA and SpB may possess immunostimulatory properties (although I say this with caution as similarities in chemical structures do not always translate to similar functions).
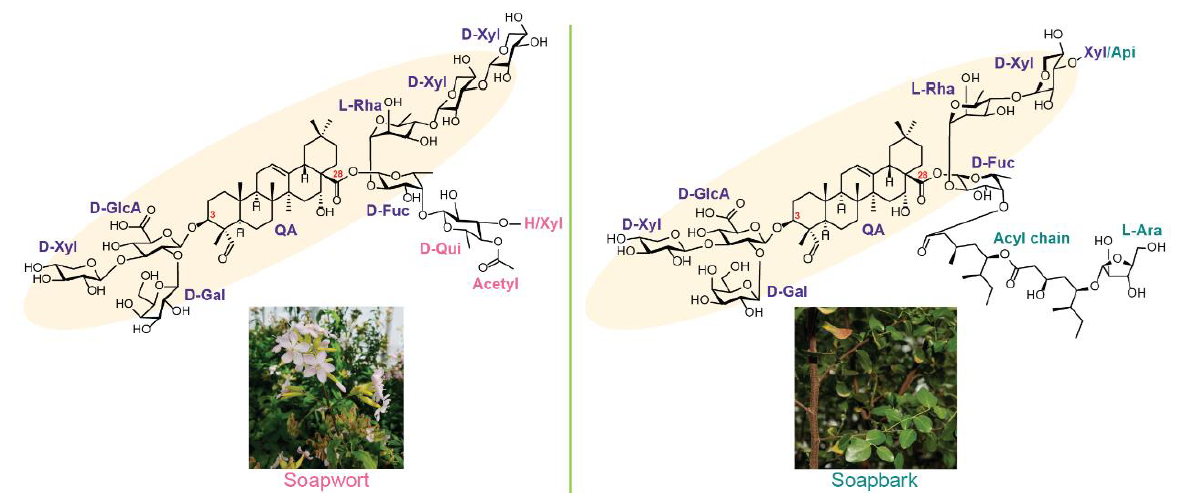
After establishing our target saponins, we performed metabolic analysis of soapwort organs to observe the accumulation pattern of SpA and SpB. This revealed flowers as the major site of saponarioside accumulation. Using this knowledge, we performed RNA-seq on six different soapwort organs and also generated a pseudochromosome-level soapwort genome. We then started to mine our sequence resources for candidate genes that may be involved in saponarioside biosynthesis through phylogenetic and co-expression analyses. After obtaining a list of candidates, we tested their functions through Agrobacterium-mediated transient expression in Nicotiana benthamiana. Overall, we discovered a total of 14 enzymes involved in saponarioside biosynthesis and reconstituted this pathway in N. benthamiana to produce SpB. Within the newly discovered soapwort enzymes, we report a non-canonical cytosolic glycosyl hydrolase family 1 (GH1) transglycosidase (TG), SoGH1, required for the addition of D-quinovose (commonly found in marine animals, but rarely found in plants) to the precursor saponin.
Unlocking a pharmaceutical treasure trove:
So what does this all mean? Through understanding the genes and enzymes involved in saponarioside biosynthesis, we pave the way for metabolic engineering of soapwort saponins in heterologous systems, opening-up opportunities for large-scale production and biochemical studies of these biologically active saponins in the future. We also present an alternative route to produce valuable QS-saponins and their intermediates and analogues. As we gain more and more knowledge into these intricate biosynthetic pathways, we open-up possibilities for accessing and engineering these high-value natural products, and even start to design new-to-nature compounds with optimized therapeutic properties.
And to think that we gained all this from a common garden plant! What treasures will your gardens be harbouring?
References:
- Osbourn, A. Saponins and plant defence—a soap story. Trends Plant Sci. 1, 4-9 (1996).
- Reed, J. et al. Elucidation of the pathway for biosynthesis of saponin adjuvants from the soapbark tree. Science 379, 1252–1264 (2023).
- Martin, L. B. B. et al. Complete biosynthesis of the potent vaccine adjuvant QS-21. Nat. Chem. Biol. 20, 493–502 (2024).
- Jia, Z. H., Koike, K. & Nikaido, T. Major triterpenoid saponins from Saponaria officinalis. J. Nat. Prod. 61, 1368–1373 (1998).
Follow the Topic
-
Nature Chemical Biology

An international monthly journal that provides a high-visibility forum for the chemical biology community, combining the scientific ideas and approaches of chemistry, biology and allied disciplines to understand and manipulate biological systems with molecular precision.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in