Oxygen delivery in tiny packages
Published in Bioengineering & Biotechnology
_large.jpg)
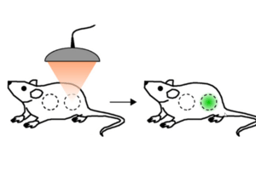
Currently the only way to rescue patients from prolonged oxygen deprivation resulting in hypoxemia — an abnormally low oxygen concentration in the blood — is either by mechanical ventilation using a tracheal tube or by oxygenation of blood outside the body1. Both strategies are fraught with complications and the former can be tricky if the airway is obstructed, not least due the narrow time window available to recover normal oxygen levels before hypoxemic consequences, such as brain damage and heart failure, occur. In the November issue of Proceedings of the National Academy of Sciences Seekell and colleagues demonstrated that polymer hollow microparticles (PHMs) injected into the blood for the systemic delivery of oxygen to rats succeeded in increasing the amount of oxygen in their blood to clinical recovery levels.
The PHMs were fabricated in a process whereby poly(D,L-lactic-co-glycolic acid) (PLGA), perfluorooctyl bromide (PFOB) and Pluronic F-68 were combined to create a PLGA/F68-rich shell with tiny pores and a PFOB rich core. Because of the oil-in-water emulsion that was created inside the core on the addition of water, subsequent freeze-drying of the microparticles resulted in oxygen gas filling the core. The authors showed that oxygen inside PHMs could be released and bind to oxygen-depleted haemoglobin (deoxyhaemoglobin) — the molecule inside blood cells that transports oxygen to every cell in the body. They did this by mixing the PHMs with deoxygenated human blood in the lab and showed that the oxygen was transferred from the PHMs to deoxyhaemoglobin, re-oxygenating the blood cells within seconds. The microparticles work on the same principle as wicking material in sports clothing — where moisture is drawn through channels against forces of gravity to keep the skin dry. Here, blood is wicked into the channels in the PLGA shell, and gas exchange occurs at the oxygen/blood interface, diffusing from high (in the microparticle core) to low (in blood plasma) oxygen content, in a process identical to the mechanism of gas exchange in the lungs. When PHMs were injected into leg veins of rats in a model of oxygen-depleted blood vessels, the oxygen content was raised to clinically important levels for approximately 10 minutes, in contrast to control animals injected with an electrolyte solution only. Furthermore, haemodynamic parameters showed that the PHMs do not cause any obstruction in the blood vessels.
The advantages of PHMs compared to other injectable systems are considerable: they can carry far more oxygen, the PGLA shell renders them more mechanically robust than lipid-based particles (tested previously by the authors2), and they are less susceptible to leakage or degradation during storage, as evidenced by their long shelf life of 60 days with no significant changes in size or gas-carrying capacity. Taken together with the fact that PHMs do not require functioning lungs to oxygenate them as previous technologies do, this means that they could be used to treat hypoxemia due to respiratory failure as a result of damaged lungs or airways.
Before patients can breathe a sigh of relief at the idea that PHMs can alleviate disorders like hypoxemic cardiac arrest or haemorrhagic shock, additional studies are required to identify and minimise any potential side effects, and to track the removal of oxygen-depleted particles from the body. Finally, although the volume injected is considerably lower than in the authors’ previous work to achieve a similar effect, a limitation of the application of PHMs is that repeated injections may be required to maintain oxygen levels, which means injected fluid remains in circulation and may need to be reduced in some scenarios2. Additionally, PHMs do not remove carbon dioxide. Nevertheless, these limitations seem to be surmountable and PHMs could provide a myriad of potential short-term therapeutic applications for critically ill patients.
HIGHLIGHTED PAPER
Seekell, R. P. et al. Oxygen delivery using engineered microparticles. Proc Natl Acad Sci U S A. 113, 12380–12385 (2016).
FURTHER READING
1. Villar, J. & Kacmarek, R. M. Rescue strategies for refractory hypoxemia: a critical appraisal. F1000 Med Rep. 1, 91 (2009).
2. Kheir, J. N. et al. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci. Transl. Med. 4, 140ra88 (2012).

_medium.jpg)

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in