Waves of combined nerve and intestine stem cells
Published in Bioengineering & Biotechnology
As with the demand for replacement organs, there is also a great need to replicate, often patient-specific, organ and tissue behaviour outside the body for the purposes of diagnosing disease, determining its mechanisms and predicting drug responses that may vary between individuals. To increase the parity between the behaviour of human organs and lab-made replicates, minimise the use of animals, and lower costs, researchers have advocated organoids as a laboratory model that presents solutions to these challenges. Organoids are a self-assembling group of different cell types that, when combined and stimulated with nutrients, spatially organize in ways akin to those of a specific organ to eventually perform at least one of its specific functions1.
In the November issue of Nature Medicine, Workman et al. combined human intestinal organoids (HIO) and enteric nervous system (ENS) cells derived from pluripotent stem cells (PSCs) — precursor cells typically derived from embryos that can become almost any cell in the body — to create a functional intestinal nervous system. The incorporation of functioning nerves builds on a previous pioneering technique of creating human gut organoids in the laboratory2. They also used this model to recreate and investigate one of the mechanisms of Hirschsprung's disease, a disorder in which the large intestine fails to develop a nervous system, causing intestinal obstruction and in rare instances short bowel syndrome.
In a departure from the engineering method that engrafts neural precursors onto the diseased or healthy tissue3,4, the researchers took a bottom-up approach and recreated the gut by recapitulating its embryonic development. They grew both intestinal organoids and nerve cells from PSCs that were then combined with centrifugation, embedded in a nutritional gel to create a three-dimensional growth system, and maintained in the lab for 4 weeks.
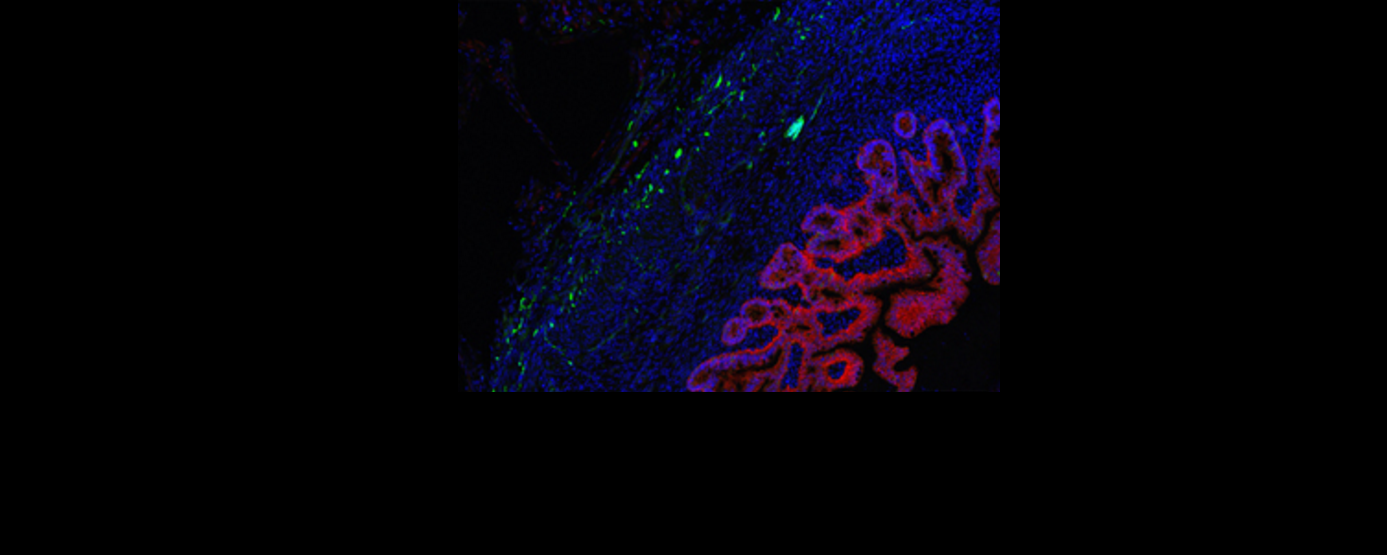
After demonstrating markers of nerve growth that were absent in constructs without the incorporation of nerve precursors, the HIO+ENS constructs were implanted in mouse kidneys (a location chosen due to the abundance of blood vessels) to test their function. Stained sections of tissue taken from the mice show that the cells differentiated — becoming specialist cells — and spatially organised during growth, resembling the structure of natural human intestine. The nerves also integrated well into intestinal smooth muscle. When explanted and exposed to electrical-field stimulation, the constructs exhibited a peristaltic like contraction — a series of wave-like muscle contractions that moves food along the digestive tract. Blocking cells common to both HIOs and ENS with methylene blue depressed pulsation in both constructs, showing that they were responsible for contraction, which was therefore independent of the ENS. However, matured nerve cells of the ENS were found to be the drivers of relaxation through an experiment where pharmacological stimulation of the ENS induced muscle relaxation in the HIO+ENS but not in HIOs alone.
Further to teasing out the respective roles of neuron and enteric precursors in the mechanism of gut motility, the researchers used the HIO-ENS model to study the molecular foundations of a severe form of Hirschsprung’s disease that results in severe depletion of ganglion nerve, caused by a mutation in the PHOX2B gene5. To this end they created induced pluripotent stem cells (iPSCs) — pluripotent stem cells generated from adult cells — with the PHOX2B mutation, and combined them with HIOs to create disease-carrying HIO+ENS organoids. There was a reduction in the expression of genes relating to nerve and muscle development. Constructs that were transplanted into mice reflected the poor genomic profile in poor muscle and nerve growth.
The creation of human intestinal tissue under the control of a functional enteric nervous system brings us one step closer to providing transplantation tissue for patients with short bowel syndrome. Its recapitulation of the human structure and function of the gut, in contrast to mouse models, and its experimental compliance means that it also provides a unique platform for a more intricate understanding of the mechanism of the gut in human healthy and diseased tissue.
HIGHLIGHTED PAPER
Workman MJ et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. (2016); published Nov 21.
FURTHER READING
- Fatehullah, A. et al. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254 (2016) doi:10.1038/ncb3312
- McCracken, K. W., Howell, J. C., Wells, J. M. & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Prot. 10, 1920–1928 (2011).
- Fattahi, F. et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 531, 105–109 (2016).
- Hotta, R. et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 123, 1182–1191 (2013).
- Association study of PHOX2B as a candidate gene for Hirschsprung's disease. Garcia-Barceló M et al. Gut. 52, 563–567 (2003).

_medium.jpg)

_medium.jpg)
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in