Platelets for the delivery of checkpoint-blockade therapeutics
Published in Bioengineering & Biotechnology

During my PhD and postdoctoral time working on animal studies, I routinely performed surgeries to remove tumours from the animals. However, local recurrence of the disease often occurred, even if I tried my best to remove all tumour tissue. We learned from the literature that the inflammatory processes during wound healing following tumour resection could promote cancer progression1. In fact, residual microtumours remain a challenging problem during cancer management clinically2, 3. We therefore decided to search for an effective strategy to prevent cancer recurrence after surgery.
We decided to leverage cancer immunotherapy to address this issue. Recently, the immune-checkpoint-blockade-based therapy targeting the programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway has achieved a significant increase in survival in patients with various types of cancer4. However, its efficacy does not come without limitations. Systemic nonspecific overactivation of the immune cells may cause severe side effects, leading to autoimmune disorders5. Insufficient lymphocytic infiltration and low PD-L1 expression may also lead to reduced response to current anti-PD-1 immune checkpoint blockade6. We thus decided to use delivery tools to achieve targeted transportation of immune-checkpoint-blockade therapeutics to the surgical site, and thereby enhance treatment efficacy and alleviate any side effects.
We looked into the toolbox of drug-delivery carriers in our lab. Eventually, we decided to apply platelets as carriers for delivering anti-PDL1 antibody (aPDL1). We have previously engineered platelet-membrane-coated polymeric nanoparticles for targeted delivery of anticancer drugs7, 8. This time we proposed to directly apply platelets as delivery carriers, inspired by the intrinsic properties of platelets. Specifically, platelets are able to migrate to the site of surgical wounds, where residual tumours may survive after surgery9. In addition, published studies have found that platelets have the capability to recognize and interact with circulating tumour cells (CTCs)10, which are shed from the primary tumour into the bloodstream and can lead to metastases.
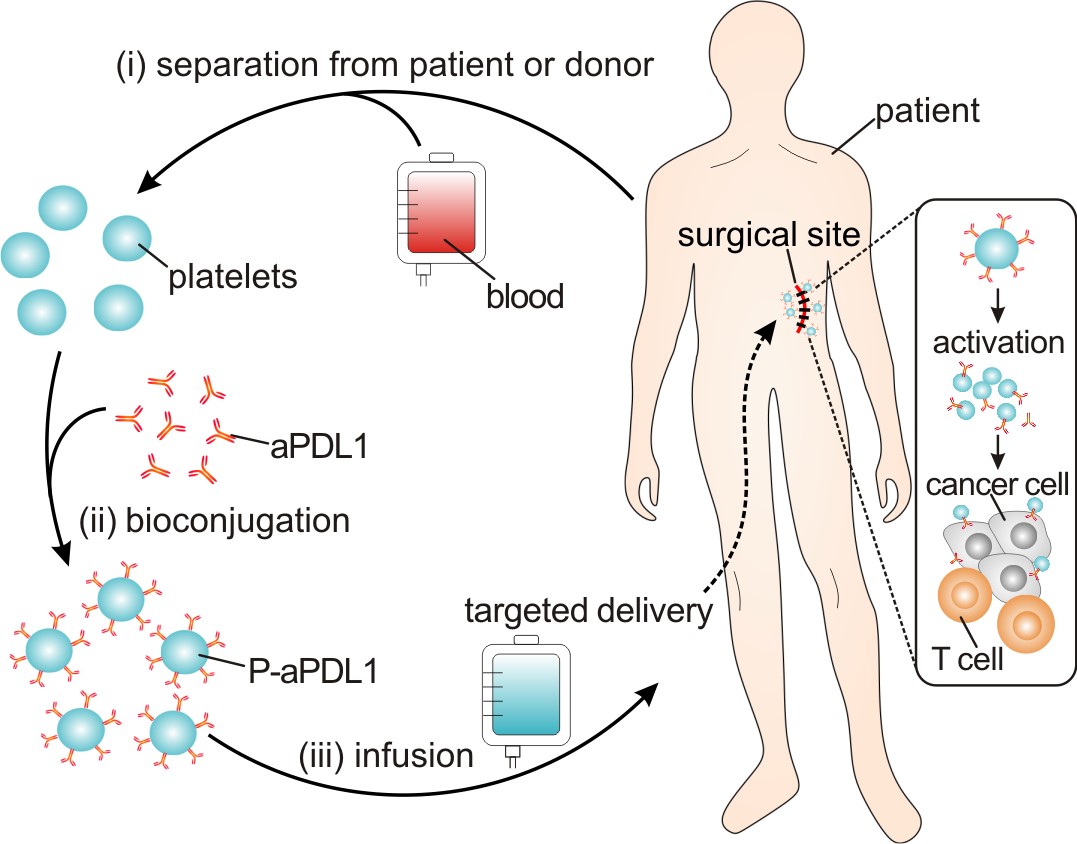 Figure 1. Schematic of the targeted delivery of aPDL1 to the primary-tumor resection site by platelets.
Figure 1. Schematic of the targeted delivery of aPDL1 to the primary-tumor resection site by platelets.
In order to test this idea, we simply conjugated aPDL1 to the surface of platelets, and hypothesized that such P-aPDL1 conjugates could be used as a preventative treatment for post-surgical tumour recurrence (Figure 1). Unexpectedly, we found that the binding of aPDL1 to non-activated platelets was highly stable, and that the release of aPDL1 could be significantly promoted on platelet activation. We reasoned that the enhanced release of aPDL1 might result from platelet-derived microparticles (PMPs), which are generated from the plasma membrane of activated platelets11. Interestingly, several pro-inflammatory cytokines were also released with aPDL1 after platelet activation. To test P-aPDL1 therapy in vivo, we used incomplete-tumour-resection mouse models, B16F10 melanoma and of 4T1 breast carcinoma, to mimic post-surgical local recurrence. Encouragingly, we found that the administration of P-aPDL1 significantly accumulated in the surgical site and prolonged overall mouse survival after surgery by reducing the risk of cancer regrowth and metastatic spread, associated with strong T-cell-mediated immune responses. In addition, aPDL1 released from platelets was clearly observed in vivo in the residual microtumour via fluorescence imaging.
In short, this strategy takes advantage of the in situ activation of platelets for the targeted delivery of aPDL1, which can substantially eradicate residual tumour cells after surgery, preventing cancer recurrence and metastasis. This platelet-mediated immune-checkpoint-blockade therapy could be extended for the delivery of other therapeutics in a bioresponsive manner12.
Our paper: Wang, C. et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1, 0011 (2017).
Reference
8. Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer Platelet‐Mimicking Nanovehicles. Advanced Materials 27, 7043-7050 (2015).
11. Nieuwland R, Sturk A. Platelet-derived microparticles. Platelets, 255-262 (2002).
12. Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater 1, 16075 (2016).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in