Platform for isolation and characterization of SARS-CoV-2 variants enables rapid characterisation of Omicron in Australia
Published in Microbiology

Australia in 2021 was a time of stark contrasts in the Covid-19 pandemic. In early 2021, Covid-19 case loads were low as we emerged from our second wave seeded by the variant clade 20F bearing the modest fitness gain of S477N. In Victoria, Clade 20F saw the bulk of transmissions in Australia and as a consequence all incoming international flights were on hold and most diverted to Sydney NSW. Whilst there were international flights, there was literally only a trickle, as Australia was maintaining a system of closed international borders accompanied with a system of Hotel Quarantine. The latter created an interesting dynamic with respect to the Covid-19 pandemic at two levels. Firstly, any breach in quarantine lead to one variant seeding infection within the community. Secondly, returning international flights brought small numbers of people but from geographically diverse sites across the globe primarily to one quarantine network in NSW. By mid 2021, the number of cases in Hotel Quarantine where the infection was acquired overseas was 3280. In a research-diagnostic partnership, a team was formed to enable genomic surveillance of those in quarantine with powerful sequencing tools like the 3rd generation Nanopore single molecule sequencing platforms and then coupled this with viral isolation and phenotypic characterisation.
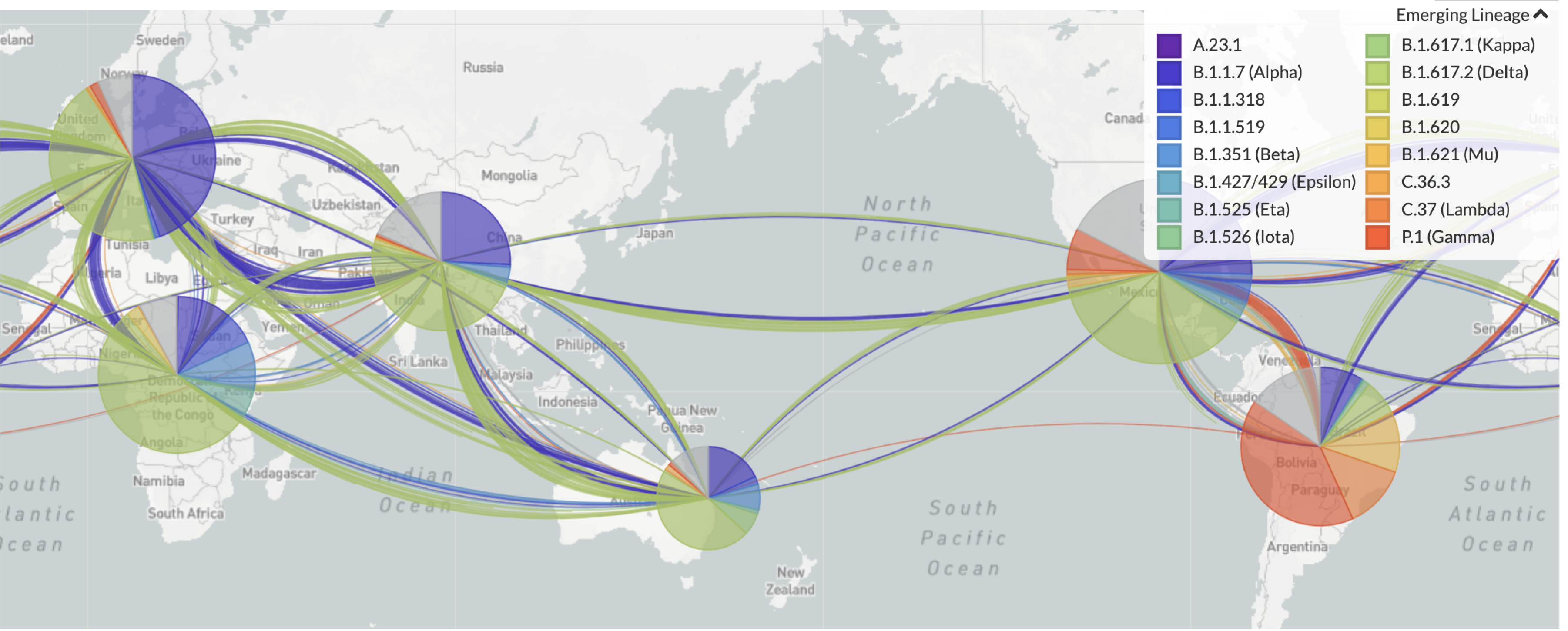
Figure 1. Summary of Variants Isolated in quarantine in early 2021 (May 2021) under Australian’s closed border policy. Image courtesy of Nextstrain at https://nextstrain.org/sars-cov-2/.
The latter phenotypic characterisation was relatively slow in comparison to genomic surveillance. Early in the pandemic isolates would be grown and titered over a few weeks and depending on the assay(s) used and the number of tests performed was often limited by the number of researchers at the bench. Whilst Covid numbers were low in Australia in 2020, the same team was responsible to screening of national blood donors through the Red Cross Blood Bank-Lifeblood 1, 2. Whilst this screening led to trials on convalescent plasma as a therapy 2, it also contributed towards testing of human antibodies pooled and purified from thousands of plasma donors in therapeutic products being manufactured by CSL Behring and their partners of the Plasma Alliance (https://www.cslbehring.com/newsroom/2020/covig19-plasma-alliance-expands-membership). This latter testing was unique, as each batch of pooled antibodies could give a population snapshot (donor pools were at times >15,000 donors) of what antibodies were in the community as a consequences of past SARS CoV-2 infection, vaccination or both.
During the early months of the pandemic, the researcher numbers were finite but it was readily apparent the samples requiring testing would exponentially expand beyond the initial testing schedules for the national blood bank. In this setting a small team needed to determine solutions in real-time. Priority was given to assays that were fundamentally low cost, easy to perform, scored by “machines” rather than operators and could be scaled to test thousands of samples per week. In parallel, diagnostic genomics teams were getting a real-time global snapshot of emerging variants in quarantine through genomic surveillance. In this latter setting, additional criteria needed to be satisfied. The first, the ease with which variants could be isolated and secondly the speed with they could be ranked based on their threat of transmission. Speed also enabled faster turn arounds and increasing the scale of testing per week.
Whilst many assays existed for SARS CoV-2, very few assays were rapid, low cost and could be scaled into the thousands per day. Isolating virus was also problematic, as often samples that were identified as key variants through viral WGS, were low in viral load. With very low numbers within Australian quarantine, it was very much a case one sample gave you one chance to isolate and characterise a key variant. Key to solving these issues is what we refer to as using the right viral cellular substrate. A cell where the infection could self-develop in a hands-off manner and a cell that could isolate virus with sensitivity approaching that of diagnostic PCR. Through genetic engineering of cells with the known receptors for SARS CoV-2 (ACE2 and TMPRSS2) using a cellular canvas already known to have a dampened response to viral infections (the Hek 293T line), the team screened hundreds of cellular clones to finally find a cell that was highly sensitive to SARS CoV-2 infection. This cell clone satisfied the criteria above. Following infection, the cells would succumb to infection in a dose dependent manner. The more virus, the greater levels of replication and in this setting cells were drawn into large balloon like structures called viral syncytia (see Movie S1 & Fig. 1). In brief, viral syncytia are formed as a consequence of an abundance of the viral glycoprotein at the cell surface of one cell and an abundance of the viral receptor on the neighbouring cell. So the viral machinery that enables a viral membrane to merge with a target cell, mediates the merging of an infected cell with its neighbour. Whilst real-time imaging of this process looks chaotic, we were able to quantify the process with high precision. Prelabelling of nuclei in live cells and machine counting after overnight culture revealed a simple, cheap, scalable method to determine the level of viral infection. The more virus, the more syncytia and in turn the syncytia would “vacuum” the surrounding nuclei in a predictable manner that could be simply enumerated by a microscope and then computer counting the number of nuclei left. The system was primarily hands off. You added the virus and then the culture would self-develop the following day. You literally just read the plate. Cheap, rapid and could be scaled. It tick the right boxes that were needed.

Figure 2. The viral syncytia “vacuum bag”. In the upper image there is no virus and numerous cell nuclei labelled in red. The next image is low level viral replication where small syncytia have formed and have “vacuumed” neighbouring nuclei reducing the field of view nuclei count. The last image large syncytia have “vacuumed” the entire field of view of nuclei. The power of this process is that it is fast (overnight) and self-developing (can be simply done at low cost and at scale). The real-time process can be observed and described below.
Whilst this broke the back of the exponentially increasing number of samples that were entering the laboratory for testing, its use with genomics surveillance then evolved into what we referred to as a sentinel network for variant analysis. In early 2021, isolates were arriving via very low case numbers in Hotel Quarantine. In the first 4 months of 2021 alone, the team isolated 12 variants, and at the time included all VOCs and VUIs. Many were from only one nasopharyngeal swabs with very low viral loads (Diagnostic Ct value of >30). The key to their isolation, was the same cell line used in high content screens was also key to sensitive recovery of viral isolates with limiting virus present. In estimates during the Delta wave in Australia, diagnostic swabs that were frozen within 24 hours of diagnosis could be used to isolate over 75% of isolates with a PCR Ct value of greater than 30. In detection of viral activity alone within a diagnostic swab, this was approaching the sensitive of diagnostic PCR, with isolation of virus from samples with diagnostic PCR Ct values of 37.
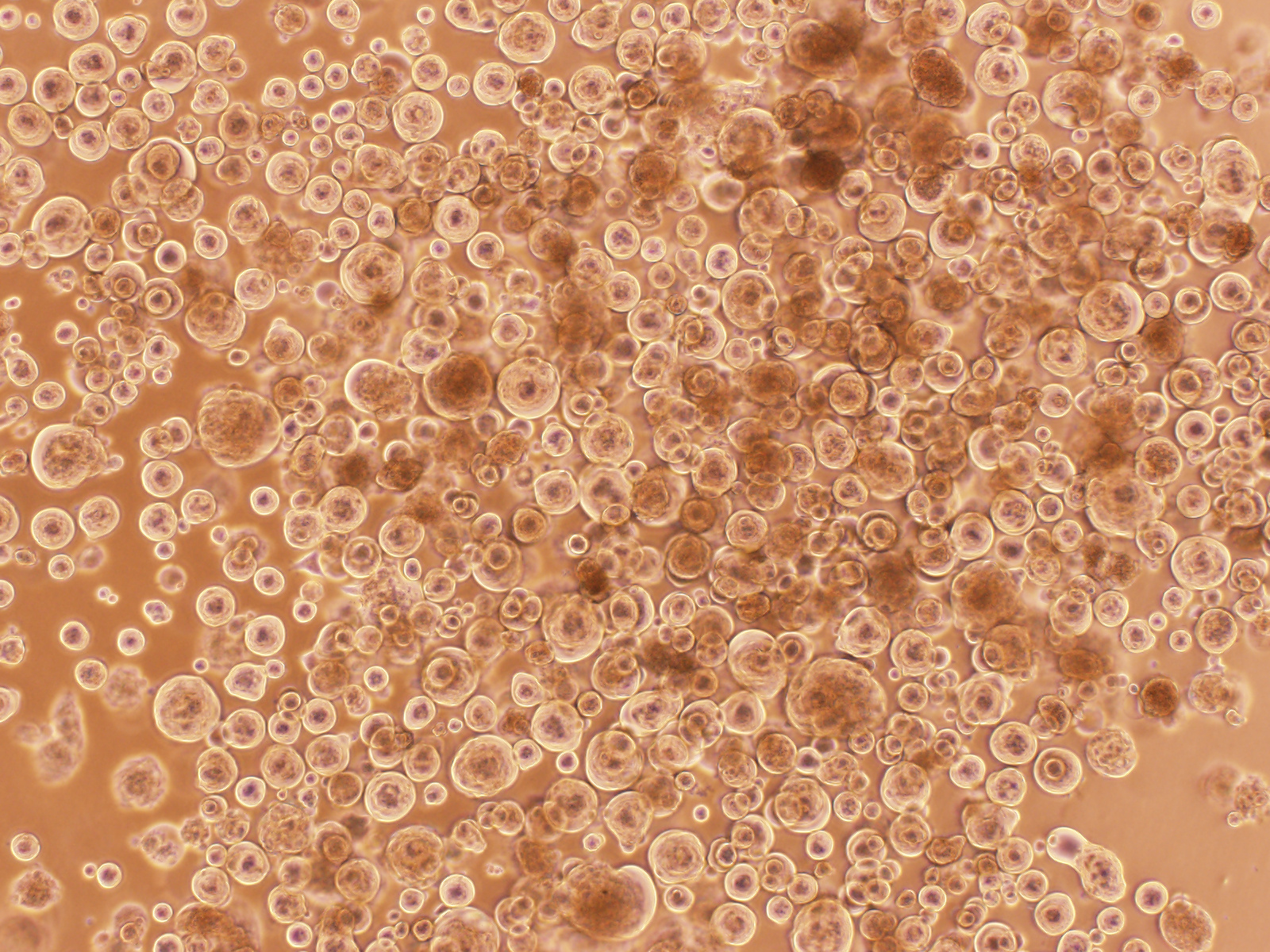
Figure 3. “Covid balls” emerging from a Delta AY39.1 primary swab sample. As with high content assays, the formation of viral syncytia would be a tell-tale sign of viral replication. For primary swabs, the cultures would reveal infection as a collection of large round syncytia we often referred to as “Covid balls”.
By the end of 2021, 12 key variants were extensively characterised. Their rank in evasion from antibodies was consistent with what was known. Their infectivity per viral load was also, with Delta isolated from primary swabs observed to be at the level of infectivity of virus expanded within the laboratory. Fortunately, in Australia this accelerated our vaccine response. Delta was a wake-up call to get moving on the vaccine front. In mid-November, with interest we observed the emergence and discovery of Omicron BA1 by teams in South Africa. At this time Australia had built its plan to relax restrictions and open its borders based on the dominant variant of the day, Delta. By the end of November, the team was isolating Omicron BA1 from one of the first cases in Australia. This would be the 13th variant major variant isolated for 2021 and the last. Within one week, the team has a snapshot that this virus would significantly challenge the current vaccines primarily in use in Australia, AstraZenca’s ChAdOx1 and Pfizer’s BNT162b2 (https://www.smh.com.au/national/nsw/inside-sydney-s-ultra-secure-lab-where-scientists-put-omicron-through-its-paces-20211210-p59gim.html). On the same day, the Sigal laboratory posted their first results from South Africa and highlighted Omicron would challenge our vaccines moving forward 3. The following week, the team consolidated their efforts to large clinical therapeutic panels and panels of serum that were the peak responses of combined convalescent recovery from early in the pandemic with two dose vaccination and three dose vaccine schedules of a cohort of research and health care workers. For clinical antibody based therapeutics, Sotrovimab was one of the only options left in the clinical cupboard 4. For the best of the best responders that with either convalescent immunity following infection and vaccinated or three dose vaccinated, the results were the same, a 16-fold drop in potency. Whilst slipping through antibody responses was one trick the virus had evolved to do, it had also changed on another front: the way it was entering cells and tissue. Unlike Delta and variants prior, Omicron no longer followed an efficient path of entry using TMPRSS2 to enter cells. Rather it was using an entry pathway that resulted in a tropic shift towards the upper respiratory tract of the bronchus 5.
The end of 2021 started a new chapter in the pandemic. The virus as we knew it had changed it stripes. Globally Omicron resulted in a peak of global infections that was beyond any wave we had seen before. Fortunately, whilst many that were vaccinated became infected, the high global case rates did not translate to the prevalence of Covid deaths we had observed in prior waves. Many aspects of Omicron and related lineages are still presently unknown. How Omicron variants have been so successful at transmission without efficient use of TMPRSS2 is one area that many of us are looking at closely. That and how changes to entry pathways influences not only transmission but important disease severity. In 2022, we are really in a new chapter of variants. If any variant is worthy of calling a new strain, Omicron and sub-lineages thereof are.
References Cited
- Tea, F. et al. SARS-CoV-2 neutralizing antibodies: Longevity, breadth, and evasion by emerging viral variants. PLoS Med 18, e1003656 (2021).
- Writing Committee for the, R.-C.A.P.I. et al. Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA 326, 1690-1702 (2021).
- Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654-656 (2022).
- Kozlov, M. Omicron overpowers key COVID antibody treatments in early tests. Nature (2021).
- Hui, K.P.Y. et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 603, 715-720 (2022).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in