Playing with Natural LEGO bricks.
This Behind The Paper perspective is concerned with the research outlined in: https://www.nature.com/articles/s41557-018-0132-6
A fundamental problem in the discovery of biologically active compounds is the design of truly novel, biologically relevant molecular scaffolds. This challenge arises partly because of the almost infinite possibilities of compound structure which cannot be covered simply by practical synthesis. Natural products have historically served as a source of inspiration because their molecular scaffolds already provide a starting point of biological relevance. However, the use of natural product structure alone as a guiding principle is limited by the fact that natural products have been selected and evolved for their respective biological targets. In order to take advantage of the biological relevance of natural products and to create new molecular frameworks, we have broken them down into natural product fragments similar to LEGO bricks, which can be reconnected into alternative patterns not existing in nature to yield “pseudo natural products”. The resulting molecules will still be natural product inspired, however they can extend beyond the structure of natural products themselves.
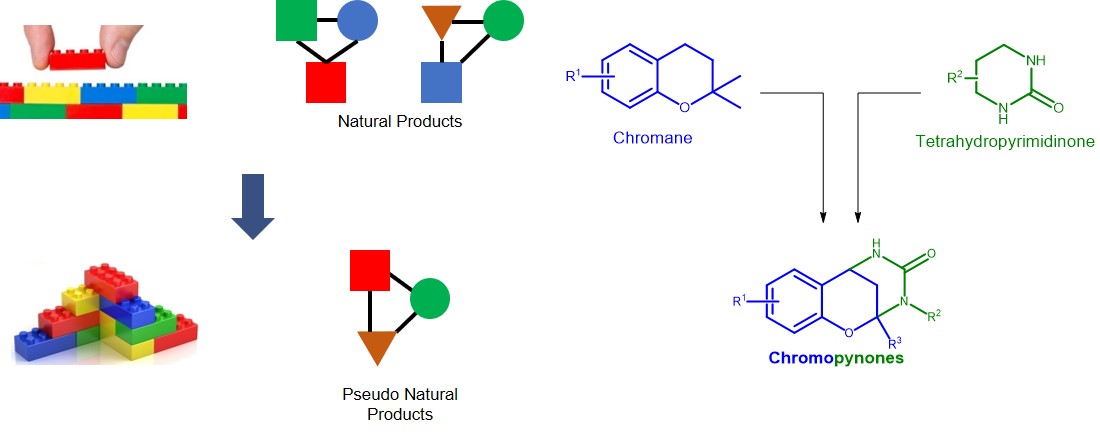
Following this principle, we designed a new class of pseudo natural products which we termed chromopynones as they combine a chromane and a tetrahydropyrimidinone fragment. Both fragments are parts of natural products which are biologically relevant e.g. antibacterials, and have not been found together in nature. Using a multi-component one-pot synthesis we prepared a collection of chromopynones. As with natural products themselves, which are tested in a variety of systems to reveal their biological effects, we tested chromopynones in a range of assays which monitor biological processes, not just individual targets. These pseudo natural products restrict the ability of cancer cells to take up glucose, on which they rely more heavily than healthy cells to produce energy.
Discovering that chromopynones are biologically relevant was only half the story. We wondered what the relationship between pseudo natural products and their natural product counter parts was. To find out we used chemoinformatic tools which analyse the similarity of atom connectivity patterns between natural products. This led to the discovery of a seemingly odd finding; pseudo natural products are not very natural product-like. However, chromopynones are the product of a combination which is not encountered in nature. Thus, they should be un-like any other natural product.
As simple as the LEGO analogy may be, it is interesting to find out that simple re-configurations of chemical matter can lead to new molecular frameworks which display biological activity. The idea that we can break down natural products at a fragment level and connect these fragments in alternative ways, is fundamentally connected to the way that chemists are trained to think. Further research into making different classes of pseudo natural products will demonstrate their potential in the discovery of biologically active molecules which may be used for the development of drugs or tools to study complex biological phenomena.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in