Post-approval safety studies: why they matter
Published in Biomedical Research
Background to this study
The first-line treatment for centre-involving DMO is the injection of intravitreal anti-vascular endothelial growth factor agents [1]. Longer acting steroid preparations are particularly valuable as second-line therapies when response to anti VEGF is suboptimal and include the dexamethasone implant (OZURDEX 0.7 mg, AbbVie Ltd., Berkshire, UK) and the fluocinolone acetonide implant (ILUVIEN 0.19 mg, Alimera Sciences Ltd., Hook, UK) [2]. Both preparations are licensed for the management of DMO. The ILUVIEN 190 micrograms (fluocinolone acetonide) intravitreal implant in applicator received the first Marketing Authorisation approval on 12th April 2012. As a specific obligation of the European Marketing Authorisations, the Competent Authorities required that a post-approval safety study (PASS) be performed as part of the Risk Management Plan (RMP). This study was named ‘ILUVIEN Registry Safety Study’, or IRISS for short (ClinicalTrial.gov ID NCT01998412) and was conducted to obtain further safety data after the initial marketing authorisations were granted. The study was conducted in Germany, the United Kingdom, and Portugal. Key features in terms of intended use were (1) that it was to be used in patients with chronic DMO and (2) it was designed to release a steady, low level of fluocinolone acetonide into the eye for up to 3 years.
The IRISS post-approval study was designed to address the Competent Authority requirement with the objective of assessing safety in real-world clinical practice and to identify potential foreseen and unforeseen long-term adverse effects associated with its use. Ethics Committee approval was obtained in Germany, Portugal, and the UK before the study commenced. Patients from 47 sites were recruited (11 in Germany, 5 in Portugal, and 31 in the UK) between January 2014 and January 2020. The end of the study was when the last patient had completed 3 years of follow-up post-treatment with the fluocinolone acetonide implant. A total of 562 patients were enrolled, and a total of 695 eyes were treated and completed follow-up visits [3].
Challenges from a design and data collection perspective
The IRISS study was designed to capture all diagnoses and included both patients with DMO and non-DMO (e.g., cystoid macular oedema, macular oedema secondary to neovascular age-related macular degeneration, macular oedema secondary to retinal vein occlusion, posterior uveitis, other, or where no disease/condition was indicated).
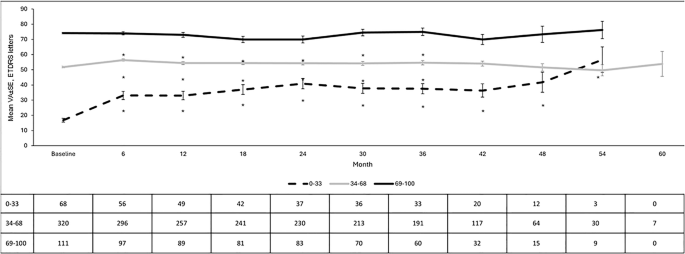
The real-world design of the study naturally presented challenges. For instance, the duration of DMO was not always captured. Both eyes could be treated, so some patients had a mix of one eye being treated or both or multiple implants being delivered. The lens status was primarily pseudophakic, but there were eyes with a phakic lens or aphakic. Measurements of visual acuity and treatment related effects that were recorded during clinical visits were extracted from clinical notes but because this was not a clinical trial visits were not at specified intervals so the number of eyes at each time point of analysis was not the same throughout. In addition to the fluocinolone acetonide implant, patients were allowed to receive supplemental treatments. On a patient level basis, the study lasted for 3 years and 6 years overall. Correction for refractive error was not mandated as in a protocol-driven phase 3 trial so visual acuity measurements that were captured from clinic notes were made according to the local clinics routine care standard. The protocol was amended to permit retrospective enrolment and reduce the original sample size during the study, after consultation with the Competent Authorities, while ensuring that the probability of detecting safety events at a true incidence rate was not compromised.
Success stories from this study
This is one of the largest and longest studies to have data after treatment with the fluocinolone acetonide implant. Several peer-reviewed manuscripts have been published in high-impact medical journals from this dataset, and it continues to be a valuable resource to explore outcomes obtained in routine care settings [4]. It is a rich database as it provides information on safety and effectiveness, as visual acuity was also assessed in patients. Increased intraocular pressure (IOP) and the development of cataract remain the primary safety signals in patients receiving the fluocinolone acetonide implant. Both risks are well documented. All safety signals in the IRISS study were consistent with those identified in pivotal trials and other real-world studies, and, importantly, were contained in the license.
Personal insight
Data were highly reassuring in the context of adverse events. Very few patients experienced significant rises in IOP or uncontrollable IOP increases. As most patients were pseudophakic cataract surgery was not an issue but even in those who were phakic cataract was not an obvious outcome. Data recordings that were performed across the wide range of clinical sites were surprisingly good and consistency of outcomes was observed despite the different geographic locations and treatment practices. An excellent community spirit and willingness to engage with the study team confirmed the interest of the retina community in the use of continuous low dose release of steroid in managing DMO.
References
[1] Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185–222. https://doi.org/10.1159/000458539
[2] Downey L, Acharya N, Devonport H, et al. Treatment choices for diabetic macular oedema: a guideline for when to consider an intravitreal corticosteroid, including adaptations for the COVID-19 era. BMJ open Ophthalmol. 2021;6:e000696. https://doi.org/10.1136/bmjophth-2020-000696
[3] Khoramnia R, Peto T, Koch F, et al. Safety and effectiveness of the fluocinolone acetonide intravitreal implant (ILUVIEN): 3-year results from the European IRISS registry study. Br J Ophthalmol. 2023;107:1502–8. https://doi.org/10.1136/bjo-2022-321415
[4] Chakravarthy U, Taylor SR, Koch FHJ, et al. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103:1072–7. https://doi.org/10.1136/bjophthalmol-2018-312284
Follow the Topic
-
Eye

The official journal of The Royal College of Ophthalmologists. Eye seeks to advance the science and practice of ophthalmology with the latest clinical and scientific research for clinicians, optometrists, orthoptists, other health care professionals and researchers interested in the visual sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Sustainability in Ophthalmology
Publishing Model: Hybrid
Deadline: Ongoing



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in