Precision Health on a kidney-on-a-chip: developing state-of-the-art microphysiology platforms
Published in Bioengineering & Biotechnology, Materials, and Genetics & Genomics

Having very recently written up on Nature Portfolio about the initial outcomes of our first-in-study experiments that I designed, developed, and carried out at the University of California San Francisco (Figure 1). I elaborate on omics in renal calcification, alongside histopathology, as also completed within the same project of bioengineering and mechanobiology, to lay the groundwork to bottom-up bioengineer a pathological cascade on an organ-on-a-chip instrument. This article corroborates a data science perspective that I wrote of earlier, to bridge personalized medicine and drug discovery via bottom-up omics and top-down modeling approaches, in precision healthcare.
Here, I first exemplify the underlying experimental and omics-clinical framework that guided our understanding of pathological biomineralization, which occurs at the renal papillary tip of clinical patients – and recapitulated it on a kidney-on-a-chip instrument (Emulate. Inc) – that subsequently led to our in-lab functional assays to detect disease biomarkers. We used a human kidney proximal tubule-on-a-chip instrument as a generic model and housed the top and bottom compartments with renal patient derived macrophage cells, based on first principles to recreate a pathological environment via functional assays thereafter. These devices can be seeded with renal endothelial or epithelial cells and are hitherto referred to as a kidney-on-a-chip instrument within this context.
The secondary aim is to propose a new framework to recapitulate patient pathology on a state-of-the-art organ-chip instrument to facilitate personalized medicine and precision diagnostics for clinical patients with renal calcification, as a natural next step in the evolution and modernization of healthcare [Nature Medicine 2024]. The first-in-study experiments were completed at the UCSF Medical School, with clinical collaborations with the Departments of Urology, the UCSF Medical Center, and histopathology–immunohistochemistry collaborations with the Zuckerberg San Francisco General Hospital.
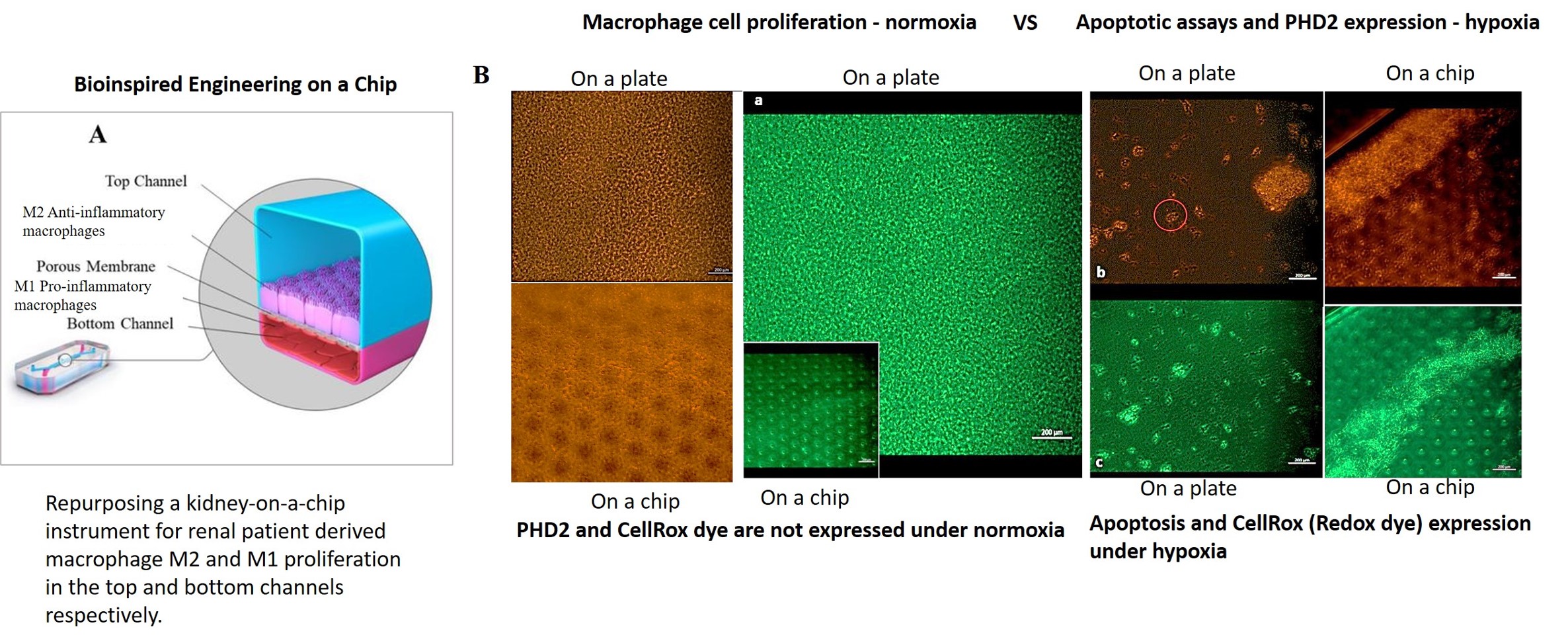
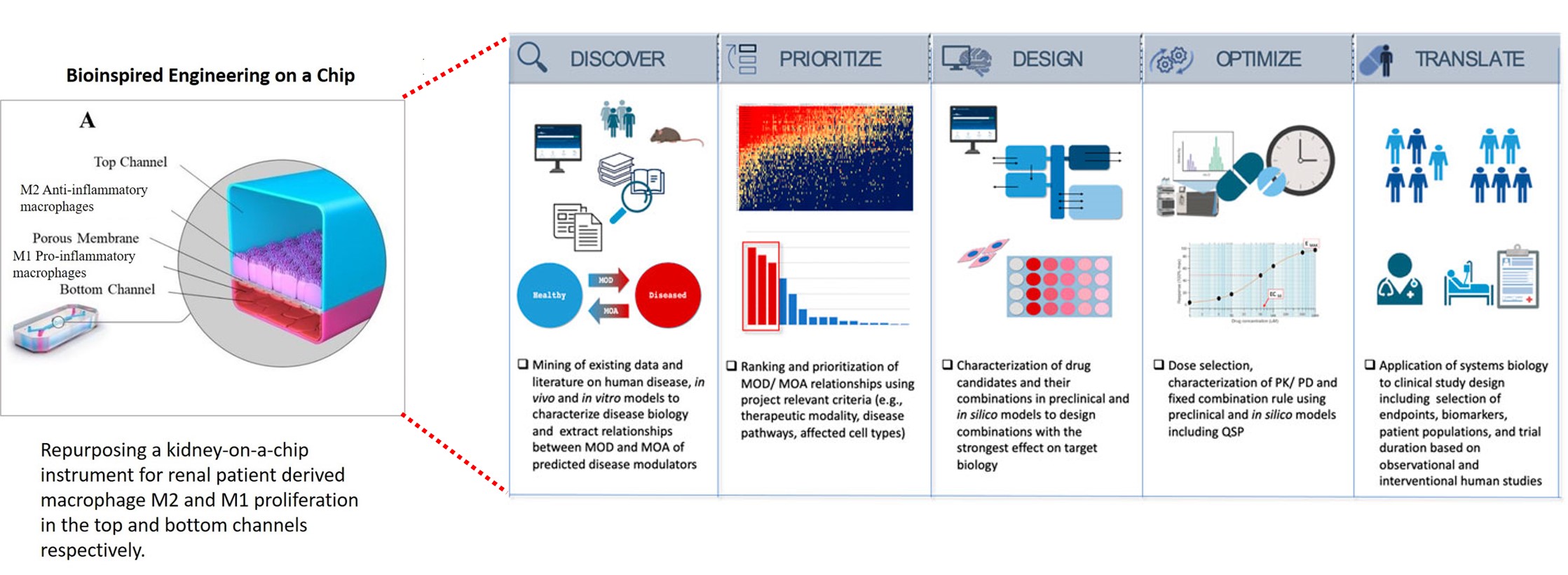
Figure 1: Recap of our study: bioinspired engineering on a kidney-on-a-chip to recapitulate biomarkers of renal calcification. A) Repurposing a kidney-on-a-chip instrument for renal patient-derived macrophage M1 and M2 proliferation on the top and bottom channels of the organ-chip instrument. B) a-c: The confluence of the macrophage cell lines was well supported on the organ chip instrument surface and on the tissue-culture plates: the cluster-forming cells are motile. Apoptotic assays were conducted on a chip and a plate to emulate bioinspired oxidative stress to observe the expression of the biomarkers of prolyl hydroxylase domain 2 (PHD2) and CellRox dye (orange and green, respectively). The morphology of the cells dramatically changed from miniature, motile M1 and M2 variants to aggregated cell clumps emitting PHD2 and CellRox under hypoxia. Image credit: Author’s own.
Core theme: Is there a biological switch at the renal tip that triggers the onset of calcification?
The key question of our study being ‘Is there a biological switch at the renal tip that triggers the onset of calcification?’ Thus far we know that no single theory has provided a simple understanding of human kidney stone formation due to the variety and complexity of calcification [Duffield 2014, Rose 2022], although the observed clinical mechanisms of kidney stone formation are primarily driven by oxidative stress and endothelial injury [Coe 2010, Saenz-Medina 2022]. How renal calcification occurs at the tip of the renal papillae is a century-old puzzle.
Our hypothesis of the presence of a biological switch at the renal papillary tip that activates during oxidative stress-driven renal fibrosis and calcification originated with patient-derived evidence-based omics and histology studies – to support our first-in-study experimental outcomes, which too were conducted with clinical samples (Jeewandara 2023, ref 6). The initial results of our biomolecular functional assays validated our proposed hypothetical mechanism of activating the oxygen-sensor prolyl hydroxylase domain 2 (PHD2) enzyme, like a biological switch at the renal tip, in response to hypoxia, which occurs due to the hypoxia factor 1 alpha (HIF1α) protein – an upregulated master transcriptional regulator of cell response to hypoxia (Figure 1) [Arsenault 2016]. We thereby established and verified a preliminary platform of renal patient-derived cells cultured on a kidney-on-a-chip [Jeewandara 2023]. Additional research works can be designed to identify the genetic basis by investigating the expression of the EGLN1 (egl-9 family hypoxia inducible factor 1) gene corresponding to PHD2, underlying pathological cascades – to further validate the biological switch activated at the onset of pathological biomineralization.
In this article, I propose the development of a state-of-the-art cell-based microphysiological platform to determine a precision healthcare narrative, based on the patient’s clinical data. These patient-specific, personalized platforms will be guided by initial renal tissue histopathology studies, to then integrate several interdisciplinary methods, to analyze the genome, transcriptome, metabolome, and proteome of renal stone forming patients to facilitate precision medicine initiatives on a chip, for the first time.
The concept can provide a patient-data driven, personalized medicine and diagnostics platform, built on patient-derived cell types and tissue samples. Such built-in microphysiological, human kidney cell-based platforms, can provide state-of-the-art instruments to obtain a comprehensive picture of the patient’s disease biomarkers in vitro, to translate personalized diagnostics and therapeutics in vivo, for customized advances in healthcare practices.
On the road – forming a roadmap for personalized healthcare – precision therapeutics
The capacity to provide patients with a personalized overview of their own pathology on a chip to optimize their diagnosis and therapeutic intervention in the clinic, can revolutionize the trajectory of treating complex diseases, including renal calcification [Misra 2024]. The data science, histopathology, and omics backed personalized healthcare platform can be based on five key principles to create a roadmap for precision health in renal stone forming patients – with translational value for bioinspired engineering on a chip from the bench to the clinic [Azer 2023].
- Discovery – to characterize the mechanism of disease during renal calcification and identify potential mechanistic routes to reverse disease biology to restore health.
- Priority – ranking the mechanisms of disease against several drug candidates to predict their mechanism-of-action.
- Design – microfluidic platforms are ideal for preclinical drug candidate studies, to select and confirm drug moieties interacting with patient-derived tissues in a microphysiological environment to regulate the intended mechanism-of-action.
- Optimization – the optimal composition, component of ratios and dosage of therapeutic drugs suited for precision medicine and healthcare can be determined on a chip, to achieve maximum effects of treatment in clinical studies.
- Translation – the development of a clinical treatment path in kidney stone forming patients can guide the clinical study design towards engineering novel small molecule drug candidates to validate pharmacological drug efficacy in the clinic.
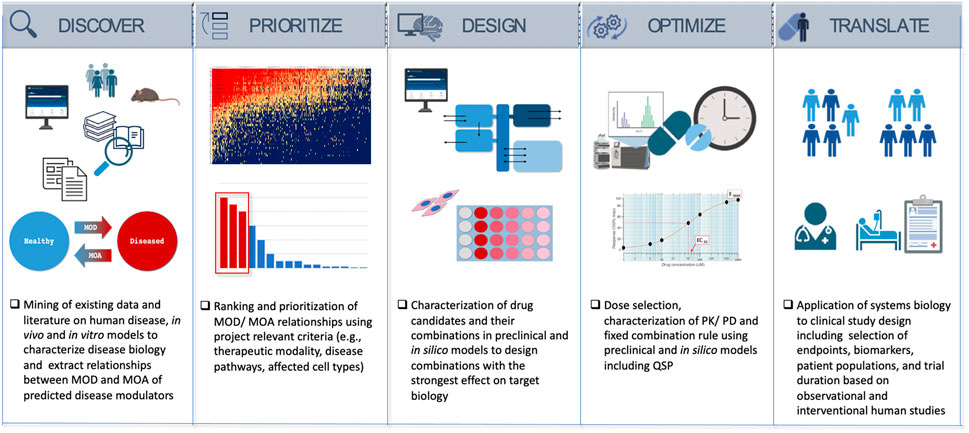
Figure 2: Information flow - Systems biology as a drug discovery and development engine in precision medicine is applicable to treat complex diseases, including renal stone forming patients [Azer 2023].
In our roadmap towards realizing precision health for renal calcification, histopathology forms an initial milestone to specify the affected nephron compartments, to assess inflammation and fibrosis characterized by immune complexes and deposits in vivo. Our work has highlighted the impact of histology studies at the renal tip of clinical subjects undergoing renal calcification, to subsequently inform the development of functional assays on an organ-on-a-chip instrument to investigate similar biomarkers of interest (such as fibrosis and renal injury) [Jeewandara 2023]. Another milestone on the roadmap of personalized healthcare includes investigating drug-pathway associations to determine toxicity and multi-drug interactions in systemic disease, to assess secondary drug toxicities that can be emulated on an organ-on-a-chip instrument [Azer 2023].
Machine learning methods can be integrated to research frameworks to ascertain potential drug-pathway associations to first determine biological trajectories and obtain a clear picture of drug-pathway interaction pairs to devise precision therapeutics in renal calcification [Wang 2020]. Machine learning algorithms can offer top-down modelling approaches to inform rational drug design with scope for clinical translation. These top-down modeling approaches can simulate the following factors, prior to conducting studies in experimental molecular medicine on a microfluidic device.
- Drug-drug similarity to assess small molecule drugs that can attenuate disease biomarkers,
- Drug-disease associations to circumvent renal calcification,
- Detect pathway-pathway similarity of multi-drug compounds
- Pathway-disease associations to develop drugs that can hinder specific pathways such as oxidative stress associated pathological biomineralization, and
- Pathway-related gene expression to substantiate the genotype of interest underlying renal calcification.
Initial milestone – identifying pathological biomarkers of renal calcification with histopathology
We analyzed clinical cohorts of patient renal papillae tissues obtained via nephrectomy (n = 34) from the UCSF Medical Center and the Department of Urology alongside patient pathology data reports. These data were categorized as stone-formers (SF) vs. non-stone formers (NSF) to examine the histopathology and genetic (bulk RNA-sequencing) composition of patient samples in the two groups. Cross-sections of the renal papillary tip were sectioned, mounted, and stained at the Zuckerberg San Francisco General Hospital and Trauma Center, to primarily assess the biomarkers of renal calcification via histology with Alizarin Red dye, collagen deposition with Masson’s Trichrome dye, and epithelial-mesenchymal transitions/fibrosis with the immunohistochemistry Vimentin-DAB antibody. These biomarkers occur at the papillary tip region, as precursors of renal fibrosis, epithelial dysfunction, and cell death prior to calcification.
For histopathology and immunohistochemistry classifications, we delineated the stone former vs. non stone former samples into a total of 19 patient cohorts with biopsies of the renal papillary tip region, of which 8 patient samples were categorized as non-stone formers, and 11 patient samples were categorized as stone-formers. The none stone-formers were clinical subjects exhibiting diseases other than renal calcification, including renal carcinoma. We conducted digital pathology multiplexing analyses via quantitative pathology software (quPath GitHub) and trained an artificial neural network (ANN) to quantify biomarkers of interest pertaining to renal calcification and kidney injury, across the cortex-to-tip region of the renal papillae among stone former vs. non-stone formers.
To facilitate ANN-assisted quantification, we trained the algorithm to segment and identify the renal papillary tip region within four specific areas assigned to tissues ranging from the cortex-to-tip region, and labelled these areas the ‘cortex area,’ Z2 (zone two) area, Z3 (zone three) area, and Z4 (Zone 4) area, respectively (Figure 3). Each region assigned within the medullary-papillae region maintained specific morphological features of tissue pathology [Ho 2018], including myoglobin renal casts, inflammatory markers of reactive nuclear changes, amyloid deposition, epithelial thinning with a beaded, ropey appearance, and intratubular eosinophilic pigmented casts that were observed in our study. A few renal stone forming patients demonstrated sediments of glomerular amyloidosis, including features of glomerular amyloid balls to indicate advanced amyloidosis in renal pathology. While the glomeruli of non-stone formers indicated normal morphology, or minimal amyloid deposition with nearly normal glomeruli (Figure 3).
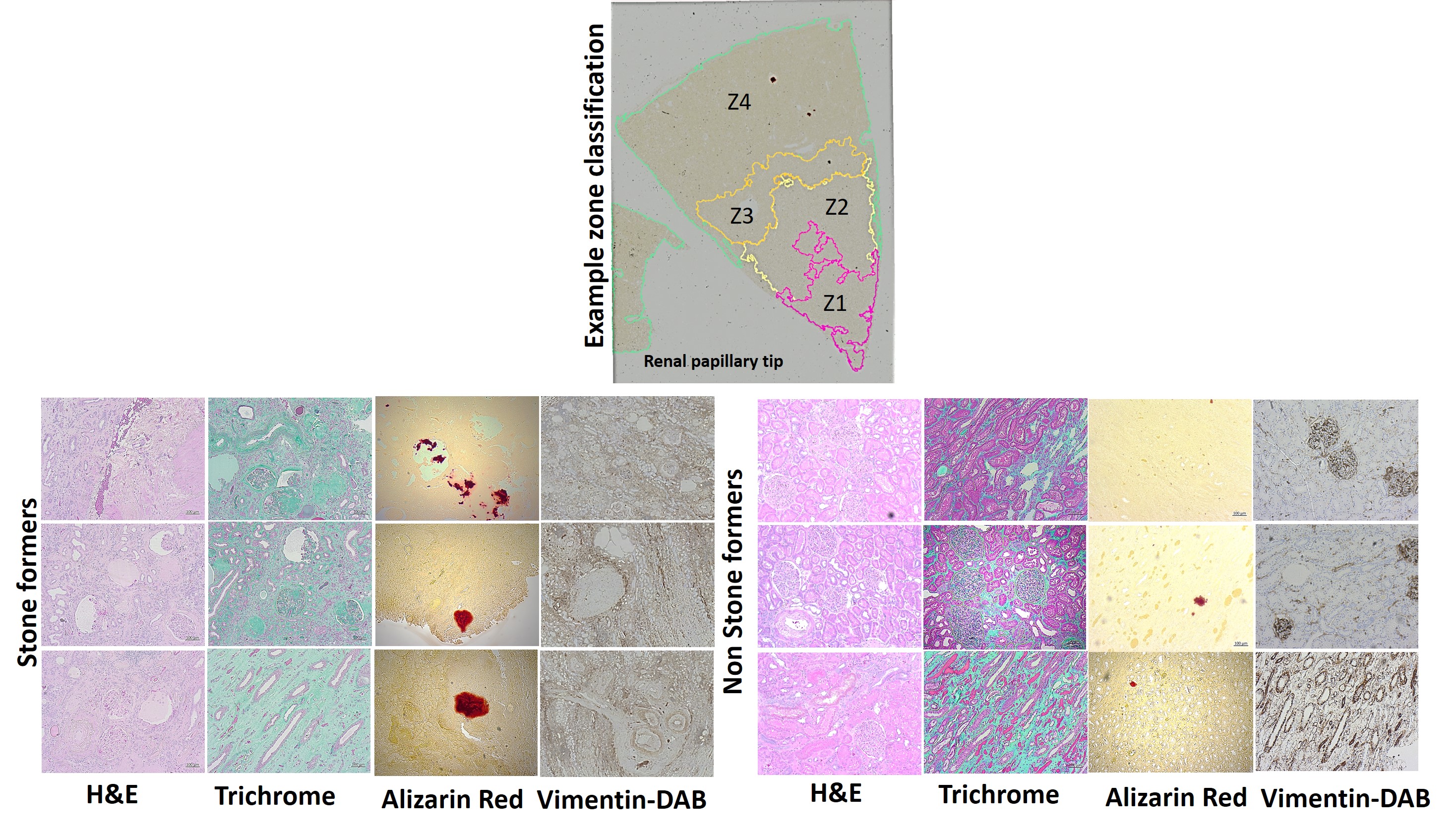
Figure 3: Histopathology data with stone formers vs. non-stone formers, the top image shows the method of zone classification used to train an artificial neural network with quPath software to quantify regions of interest (Z1-Z4) in the renal papillae. The tissue sections were stained with H&E to view inflammatory biomarkers in stone formers, including renal casts and epithelial thinning with a ropey or beaded appearance. In renal papillary sections of stone formers, the trichrome dyes indicated a higher percentage of green for increased collagen deposits, Alizarin red stained a higher percentage of crimson for calcium deposits, and vimentin DAB stained a higher percentage of brown to represent epithelial mesenchymal transitions during renal nephrolithiasis and glomerular amyloid balls in stone formers [Jeewandara 2023].
Not all cross-sections of the renal papillary tip maintained all the features of interest i.e. the cortex area to Z2-to-Z4 across the cortex-to-tip region, and as such the ANN was trained to classify the tissue sections accordingly (Figure 3). Using GraphPad Prism software, the initial measurements of digital pathology were analyzed to identify significantly higher levels of renal calcium deposits, seen as crimson aggregates (p value = 0.0017), collagen deposits that take up green colour (p value = 0.001), fibrosis that appear brown (p value = 0.0385), renal casts and inflammatory reactive nuclear changes seen with H&E images among stone former vs. non-stone former cohorts, quantified from the cortex-to-tip region of the renal papillae (Figure 4).
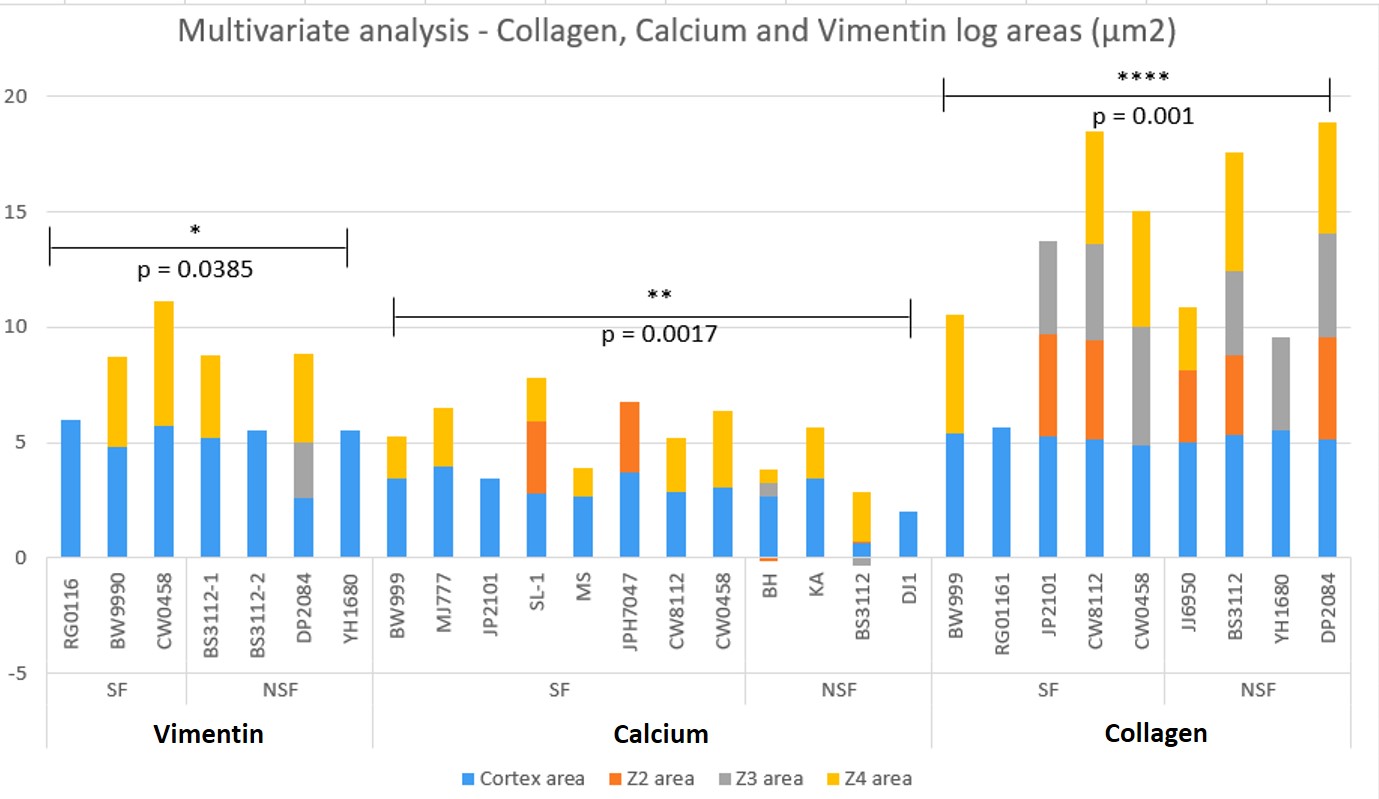
Figure 4: The quantified log values of the multivariate analyses of all samples (includes epithelial – mesenchymal transitions identified with vimentin DAB, calcium identified with alizarin red, collagen identified with trichrome) across the renal papillary cross-sections, and their p values. In this graph of ANN-derived classifications, the cortex area is assigned blue, z2 is orange, z3 is gray, and z4 is yellow. Not all tissue sections contained all classifications. There was significant variation observed in the data between stone formers vs. non-stone formers. Non-stone formers representing renal carcinoma showed higher variability with upregulated biomarkers of renal fibrosis (collagen) [Jeewandara 2023].
Next milestone – Bottom-up omics in renal calcification to form a precision health platform
How do we next integrate our first-in-study omics outcomes to inform the development of a precision health platform on a microfluidic device to address renal stone-forming pathology, and eventually guide the design and development of a small molecule drug candidate to attenuate oxidative stress-driven calcification at the renal papillary tip? For starters, the concept of precision health brings together precision medicine and diagnostics, where the core relationship can interlink personalized histopathology and genomics outcomes of patients, coupled with projected drug discovery and development procedures [Dugger 2017] – genomics is a pillar of precision health approaches [Nature Medicine 2024] and as such a primary focus in this article.
A transition to an organ-on-a-chip platform is mainly defined by problems surrounding the existing drug toxicology studies of the 21st century, i.e., what is wrong with the current approach to toxicology and pathophysiology testing? The primary issue is the limited extent to which animal models reflect the human response [Hartung 2009]. For instance, most animal models vary from human biology relative to drug interactions, metabolism, and evolutionary adaptations to protect against disease, thereby creating disparities among the species studied.
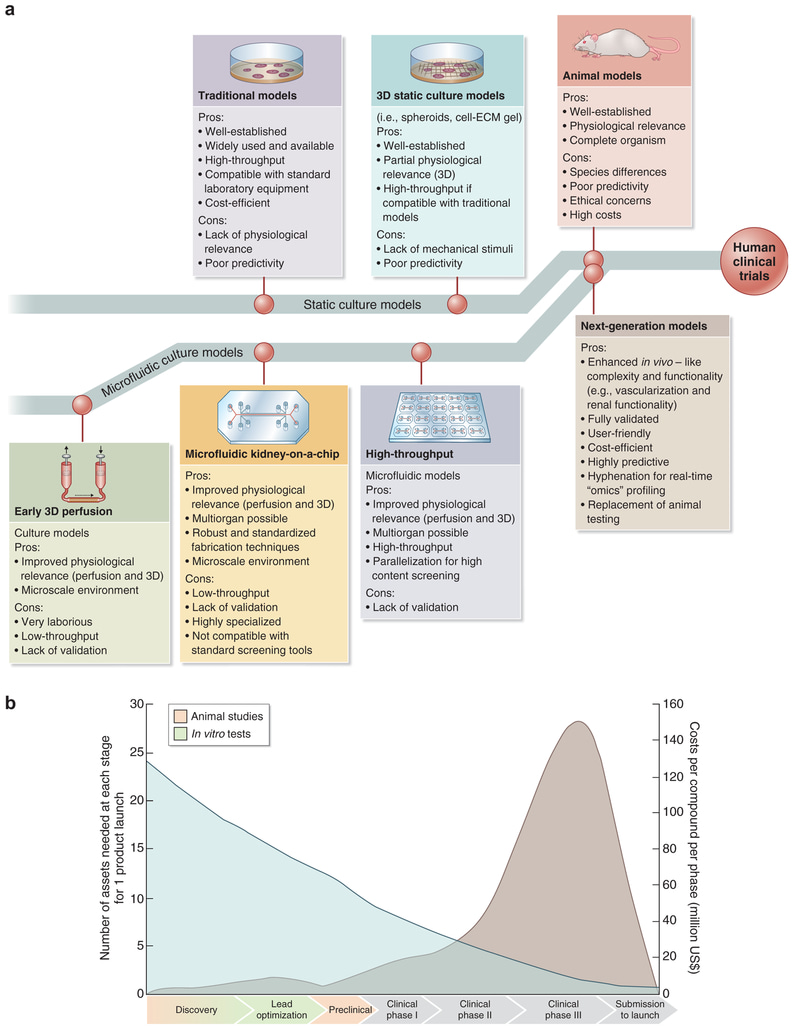
Figure 5: Future of drug testing. (a) Evolution of 3-D physiologically relevant microfluidic models for nephrotoxicity screening. ECM = extracellular matrix. (b) Drug testing challenge and dilemma [Ashammakhi 2018].
With kidney analyses thus far, microphysiological systems can develop models of human renal physiology, pathophysiology, and tissue pathology, with hitherto untapped opportunities [Ashammakhi 2018] (Figure 5). Such models surpass limits set by 2-D cell cultures and by animal studies [Hartung 2009, Jang 2013], to offer a localized microenvironment that can be regulated by the investigator, to maintain a microphysiological window to study complex diseases and drug-pathway interactions with clinically derived patient tissue samples.
The shift to precision health is well underway – allowing targeted and efficient use of resources and provides data-driven health systems that are sustainable and resilient. The field has potential to ultimately become big business, particularly in high income countries, in alignment with top-down modeling algorithms, to predict, prevent, and treat diseases [Nature Medicine 2024, Azer 2023]. The growing field of organ-on-a-chip technology, for instance, is projected to reach a $177.8 million to $6.13 billion global market by 2025 and beyond, to open new paths for multidisciplinary and translational research that meets the demands of personalized medicine [Organ-On-Chip Market 2021, Newswire 2017]. The concept of precision public health is a hallmark of precise, data-driven healthcare that informs the value of diverse and well-informed patient datasets, to gain insights and carryout optimized interventions, at the population level.
Genomics and transcriptomics offer a pillar of precision health and precision medicine
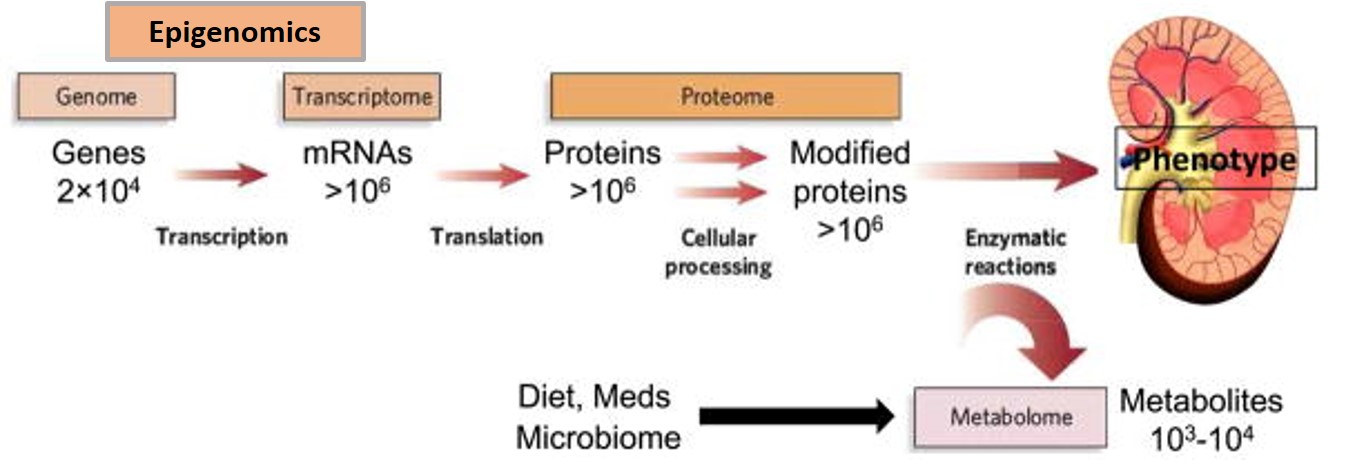
Figure 6: The flow of biological information from the genome to transcriptome, proteome, and metabolome. The epigenome conceptually resides between the genome and transcriptome. Bulk-RNA sequencing data outcomes also identify various non-coding RNAs that aren’t transcribed to proteins but have significant biological roles [Rhee 2019].
The typical laboratory flow of biological assays to extract information from the genome, transcriptome, proteome, and metabolome is as illustrated (Figure 6). Methods that analyze structural similarities between the DNA (genome) and RNA (transcriptome) are similar. In our study, we initially obtained bulk-RNA sequencing data from kidney patient renal papillary tip tissues and from biopsies, to obtain bioinformatics data (inclusive of a cDNA library and non-coding RNAs) to generate a comprehensive heatmap of genes expressed between stone formers vs. non stone formers (Figure 7). Having identified the genes expressed between the two cohorts, we analyzed the pathways enriched among the statistically significant genes: using the Database for Annotation Visualization and Integrated Discovery (DAVID) functional annotation tool.
These outcomes recognized pathways that are activated or repressed by delineating the expressed genes as either increased or decreased, in the stone-former gene sets. Using library pathview, an R package for pathway-based data integration and visualization, we obtained maps of user data (selected pathways that were upregulated or downregulated) as relevant pathway graphs. The input data consisted of gene/compound information and specific target pathways. These outcomes generated a native Kyoto Encyclopedia of Genes and Genomes (KEGG) view to represent all meta-data on pathways, including spatial and temporal information, tissue/cell types, inputs, outputs, and connections (Figure 8). The dot-plots depicted the activity of enriched signaling pathways, to view genes in the context of biochemical cascades. The enrichment dot plots were developed using an R-package to compare biological themes among gene clusters and interpret the omics data [Yu 2012] (Figure 7).
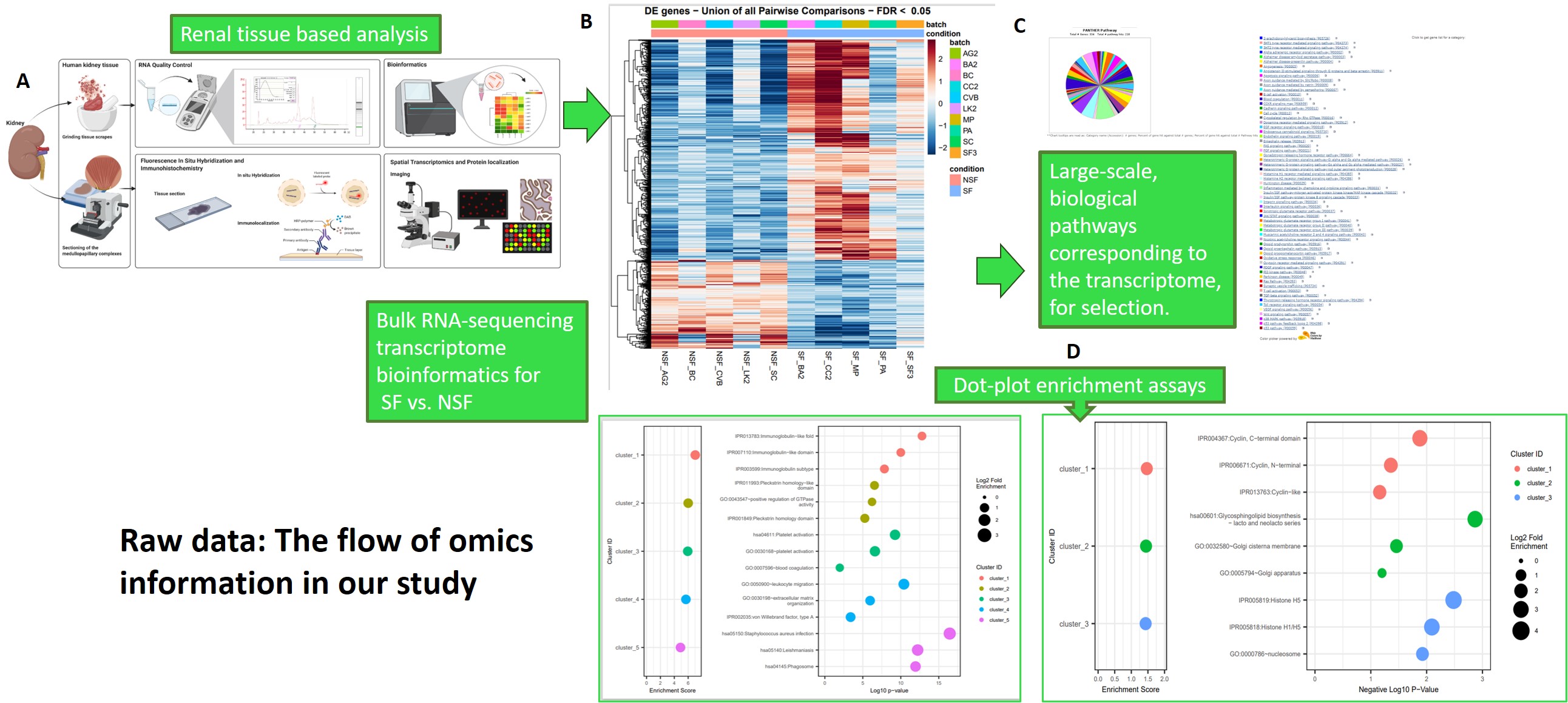
Figure 7: Raw study design: the flow of omics information in our study. A) Renal tissue-based analysis methods for bulk-RNA sequencing and fluorescence in situ hybridization to obtain B) heatmaps and spatial transcriptomics data (the latter is discussed later), C) using the bioinformatics data we then determined selective biological pathways corresponding to transcriptome data via databases (seen as a colour wheel) D) dot-plot enrichment assays to visualize the activity of enriched signaling pathways to view genes of interest (phagosomes, immunoglobin-like fold) and interpret the omics data. Image credit: Author’s own.
We also integrated DAVID-KEGG analyses and Panther 17.0 classification databases to analyze and retrieve key regulatory pathways of interest relative to the bulk RNA-sequencing omics data, including information of the corresponding genes and their associated proteins. The Panther pathway substantiated a total of 334 genes from the total transcriptomics data, to provide a colour wheel output representing diverse pathways that are upregulated relative to the bioinformatics heatmap of renal stone formers vs. non-stone formers, predominantly during pathological biomineralization. The outcomes highlighted the following pathological cascades involved at the onset of renal biomineralization (Table 1). We also incorporated biological ontologies, or gene ontology (GO) data via the PantherDB platform to annotate genes to biological processes, molecular functions, and cellular components in a directed acyclic graph structure [Ashburner 2000].
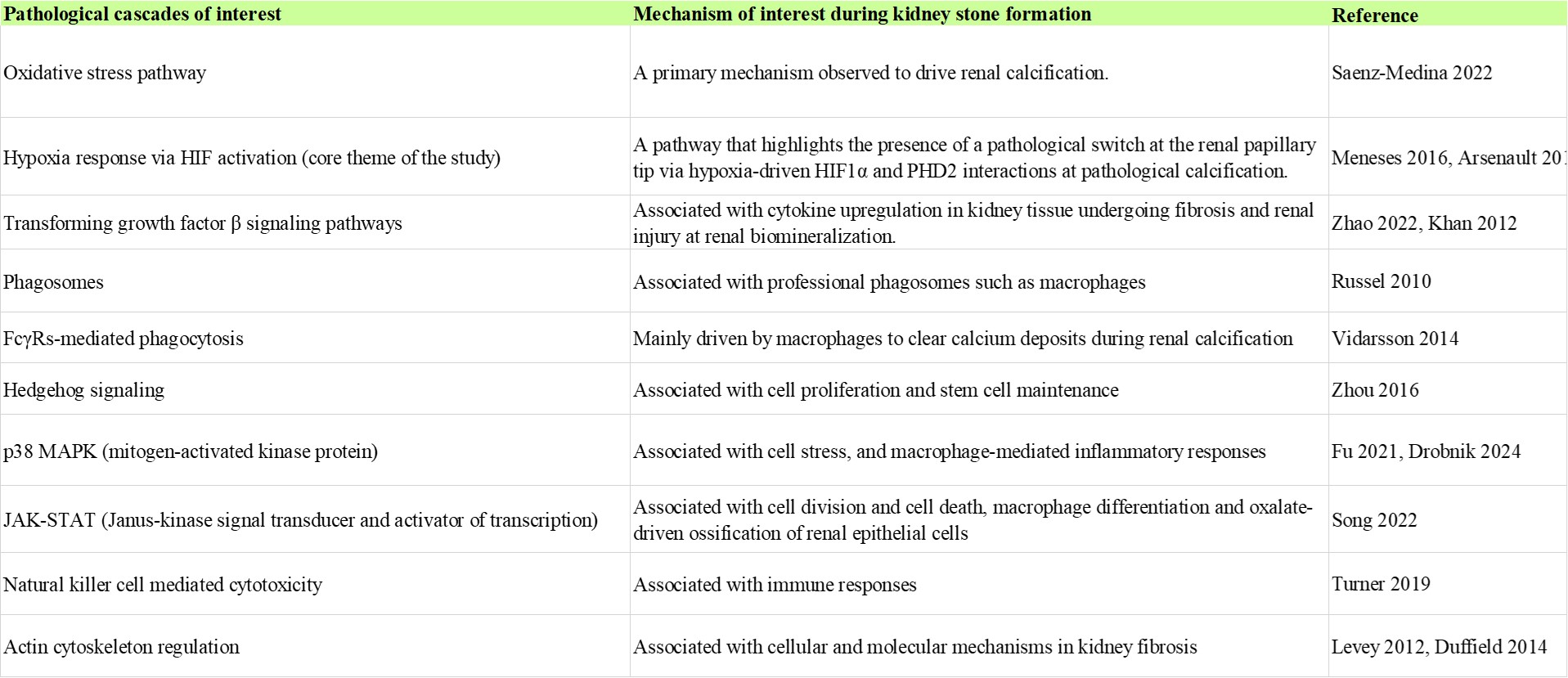
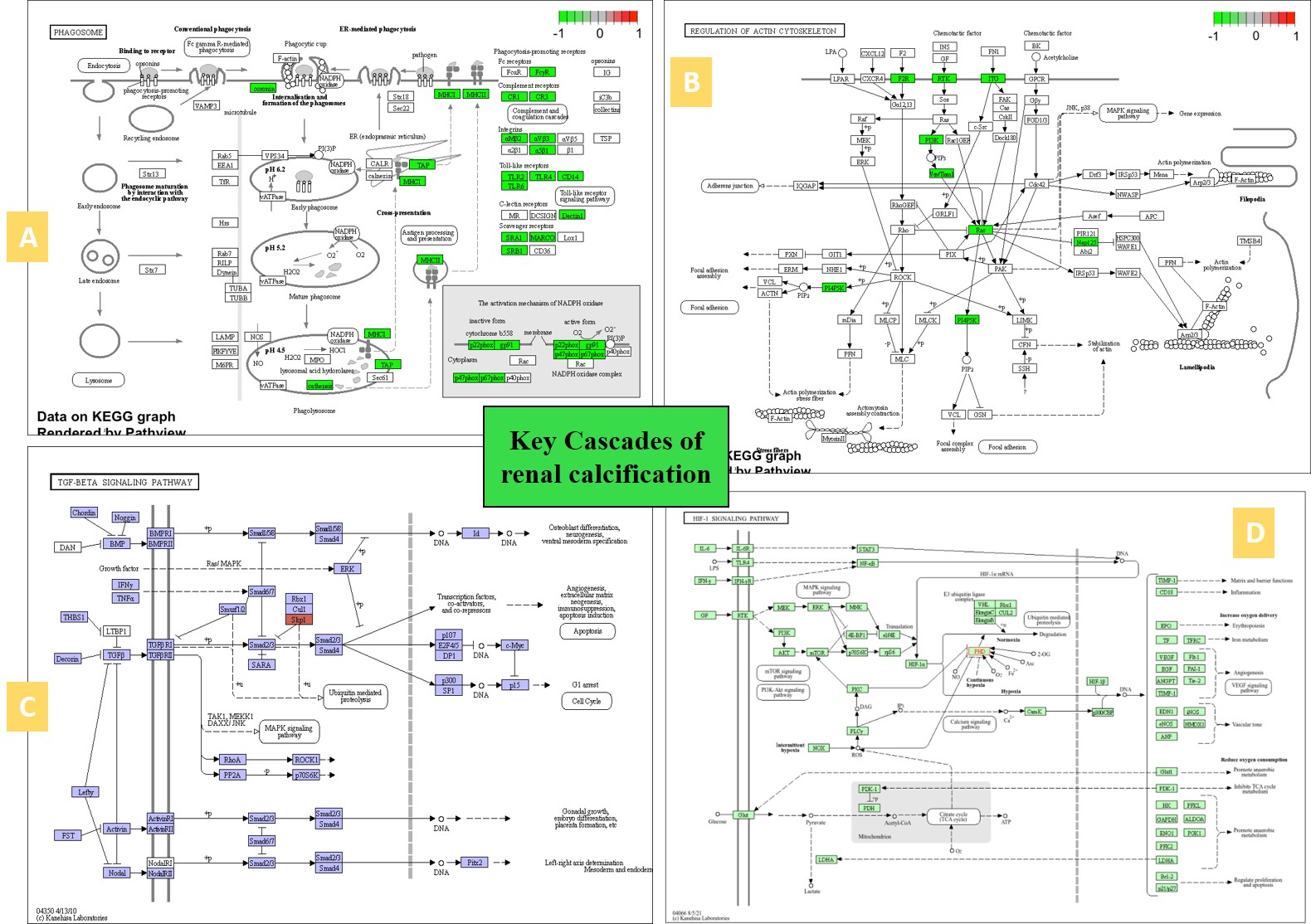
Figure 8: A snapshot of the KEGG meta-data pathways generated of the patient-derived transcriptome-driven bioinformatics to elucidate key cascades associated with renal calcification (zoom in). A) phagosome or macrophages linked to pro- and anti-inflammatory responses and renal calcium deposits, B) regulation of actin cytoskeleton associated with fibrosis, C) TGFβ signaling pathway associated with mechanical stress, D) HIF1α signaling pathway associated with hypoxia-driven cell apoptosis in nephrolithiasis. Image credit: Author’s own via KEGG.
Omics guided outcomes: establishing macrophage-on-a-chip platforms for functional assays
Our omics study outcomes of the stone former vs. non-stone former patient-derived bulk RNA sequencing data revealed a variety of enrichment plots and pathological pathways (Table 1), of which the phagosomes pathway and the FcγR-mediated phagocytosis pathway stood out to highlight the function of professional phagocytotic macrophages during kidney stone formation (Figures 7 and 8), while the p38 MAPK pathway represented another avenue of macrophage-mediated inflammatory responses [Yang 2014]. Macrophages play a significant role during hyperoxaluria-directed early renal calcification known as Randall’s plaque formation, where the M2 anti-inflammatory phenotypes ingest particulate materials to clear circulating calcium oxalate crystal deposits from renal papillary tip regions [Khan 2021].
As M2 macrophages actively engulfed calcifying vesicles to eradicate them, prolonged exposure due to a lack of clearance of hyperoxaluria, hypercalciuria or hypocitraturia-based calcium oxalate crystals, led to an increased build-up of calcium to activate circulating or kidney-resident, pro-inflammatory M1 macrophages towards initiating collagen deposition, kidney injury mediated epithelial damage, endothelial dysfunction, and pathological biomineralization, including Randall’s plaque formation (Figure 9).
Our investigations showed how the phenotype and functional polarization of macrophages can be regulated by many factors in vitro, in parallel to factors in vivo such as STATs, monocytes, cytokines, interfering regulatory factors, nuclear factors, and activating protein interactions [Mosser 2008]. Single nucleus RNA sequencing studies have additionally shown how renal calcification via Randall’s plaque is associated with embryonic-derived macrophages, instead of circulating monocytes [Yang 2023], which are likely to be stem-cell derived. In alignment with this observation, the Sonic hedgehog signaling (Shh) pathway was also upregulated in stone forming patients; a trajectory known to promote renal fibrosis in chronic kidney disease [Zhou 2017, Jeewandara 2023]. The hedgehog signaling pathway is a developmentally conserved regulator of stem cell function and the suppression of Shh signaling is associated with decreased macrophage infiltration [Gao 2010].
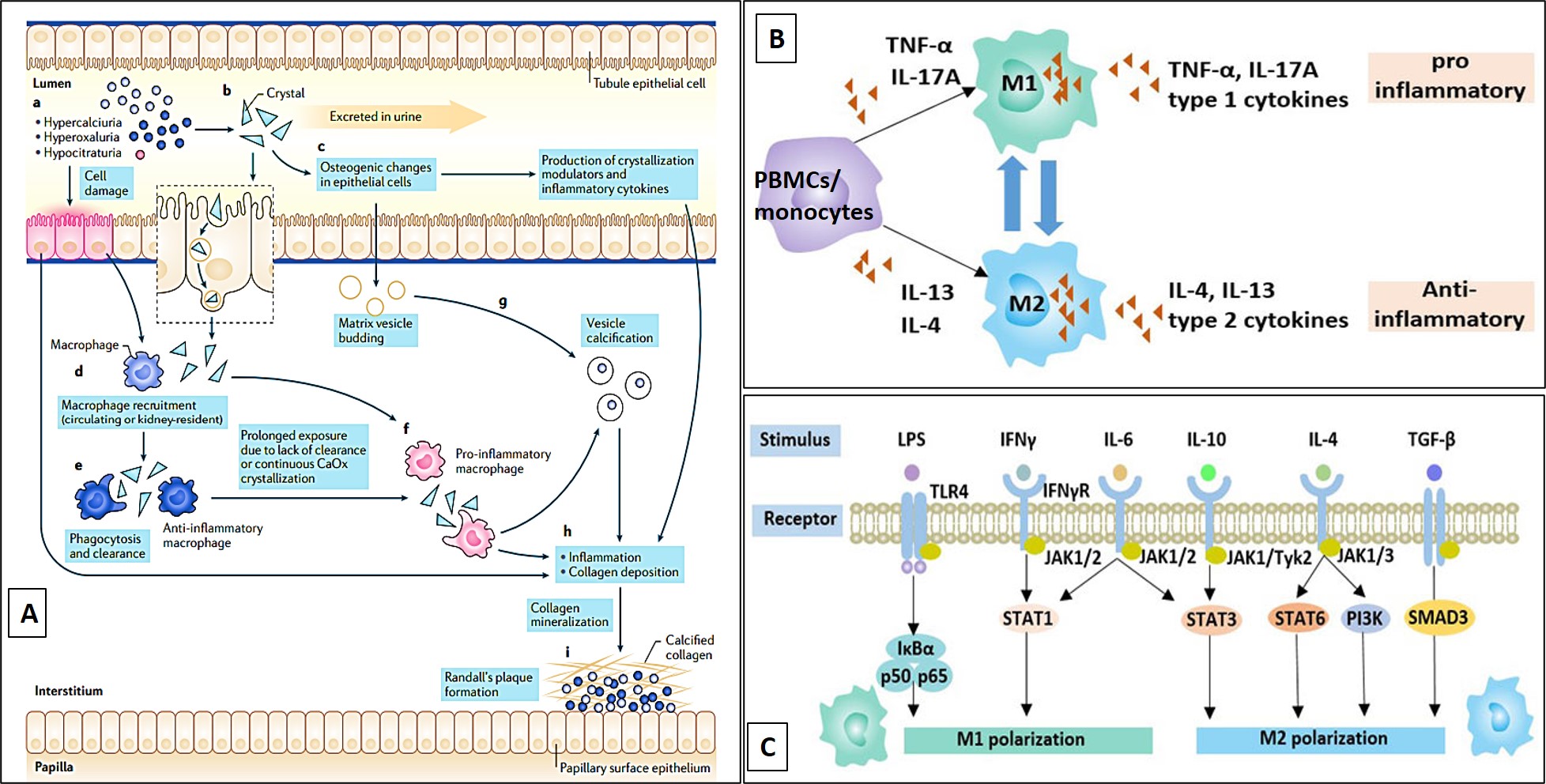
Figure 9: A) The proposed role of macrophages during Randall’s plaque formation in vivo (M1 pro-inflammatory role) and during the phagocytosis of renal calculi (M2 anti-inflammatory role). B) Peripheral blood mononuclear cells (PBMCs) can be differentiated to the M1 pro and M2 anti-inflammatory macrophage types in the presence of type 1 and type 2 cytokines, in vitro and in vivo [Khan 2021]. C) A snapshot of the JAK-STAT pathway that comes together to affect macrophage polarization in vivo [Mosser 2008].
Based on the omics outcomes, our bioinspired engineering investigations led to the differentiation of M1 and M2 macrophage cell types in-lab for the first time. To recap our work, we used renal patient derived whole blood cells of stone-formers and non-stone formers, to isolate lymphocytes and form the precursor peripheral blood mononuclear cell types (PBMCs). These PBMCs were first incubated with cytokines (TNFα, IL4) in the culture media, to differentiate them towards M1 and M2 macrophage types in a specific microenvironment within a defined timeframe.
In the presence of interferon γ cytokines and granulocyte macrophage colony-stimulating factor (GM-CSF), for instance, the cells differentiated to form M1 macrophages to promote inflammation, while incubation with interleukin 4 and macrophage colony stimulating factor (M-CSF) converted macrophages into the M2-type to inhibit inflammation (Figure 9) [Ushach 2016]. The capacity to regulate M2 polarization in the lab therefore has therapeutic value [Taguchi 2021]. We identified the macrophages differentiated in the lab by using fluorescence-activated cell sorting to distinguish between the two types of polarizations [Jeewandara 2023].
Our first-in-study experiments facilitated the differentiation of motile, and miniature macrophages for growth on a kidney-on-a-chip (generic model) instrument, where we cultured M1 macrophages on the top channel of the instrument, and the M2 macrophages in the bottom channel under adequate culture conditions, to enable the regulation of professional phagosomes in a microenvironment (Figure 1). In this way, we established and verified a preliminary platform of macrophages cultured on a kidney-on-a-chip instrument, to facilitate the following studies for their projected completion [Jeewandara 2023] -
- Develop functional assays on a chip with patient derived cell types to delineate immune biomarkers inherent to omics-identified pathways (e.g. p38 MAPK) associated with macrophage cell types during renal calcification,
- Identify the phagocytic behavior of macrophage cell types in the presence of patient biopsies on a chip, with biopsies containing inherent birefringent calcium oxalate crystals,
- Identify hypoxia-driven pathological mechanisms of biomineralization and the activation of a biological oxygen sensor on a chip (as already completed, Figure 10) [Jeewandara 2023].
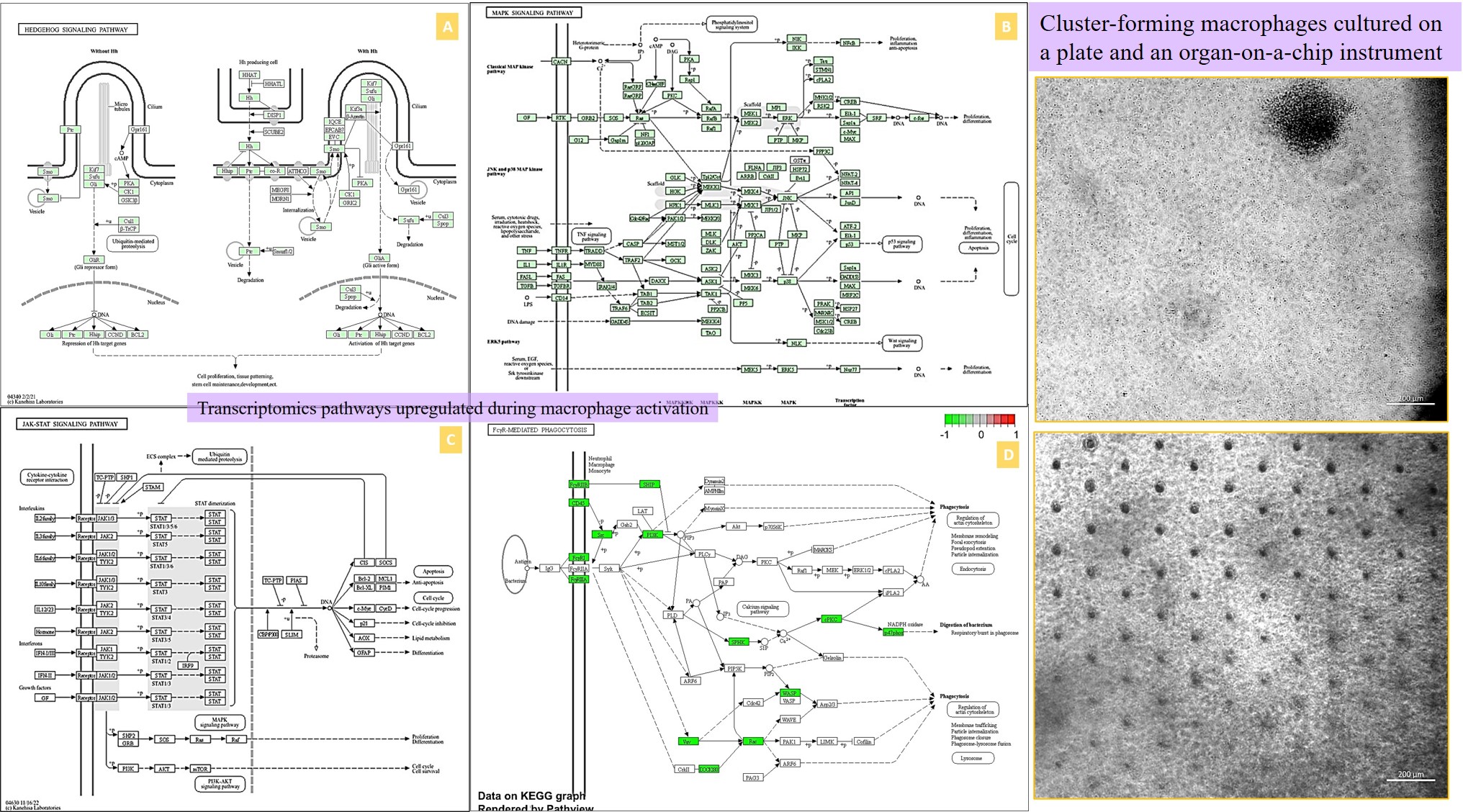
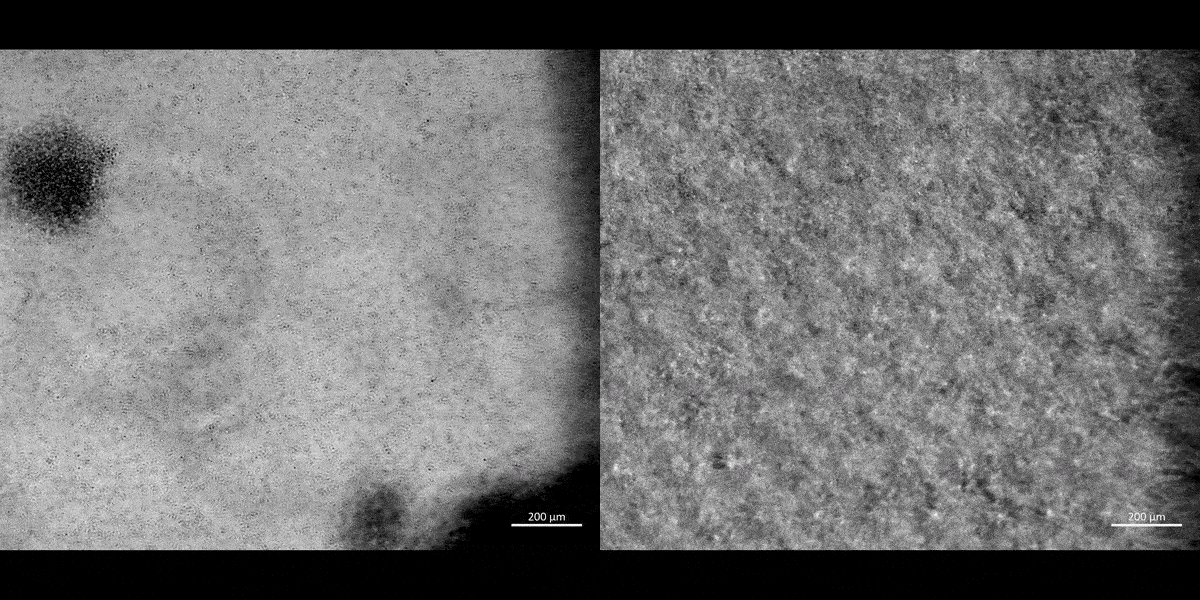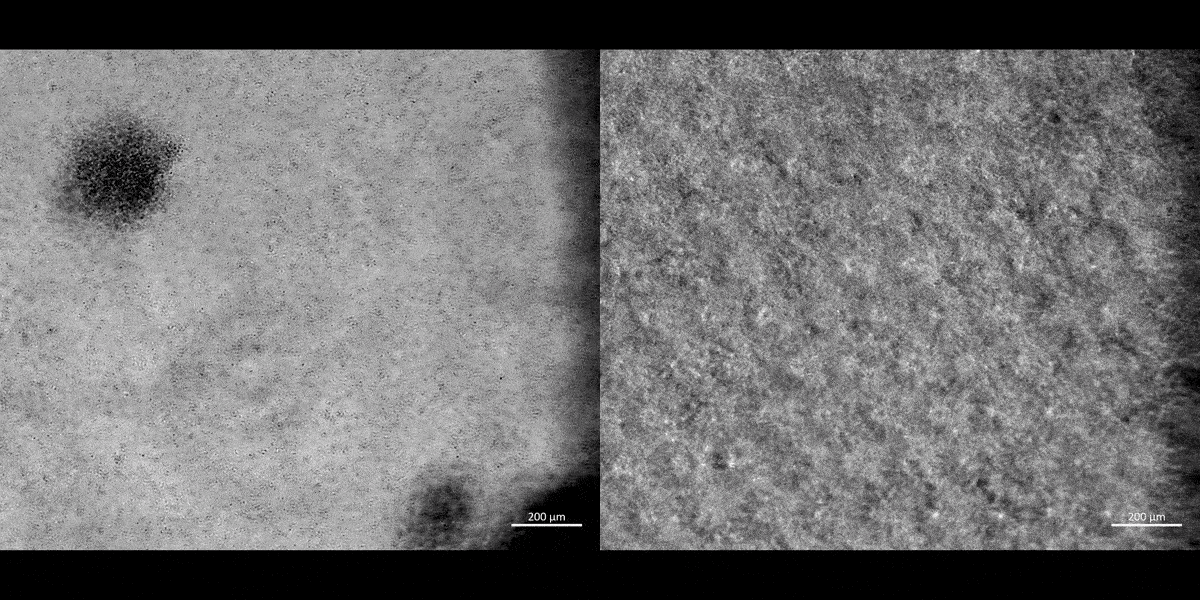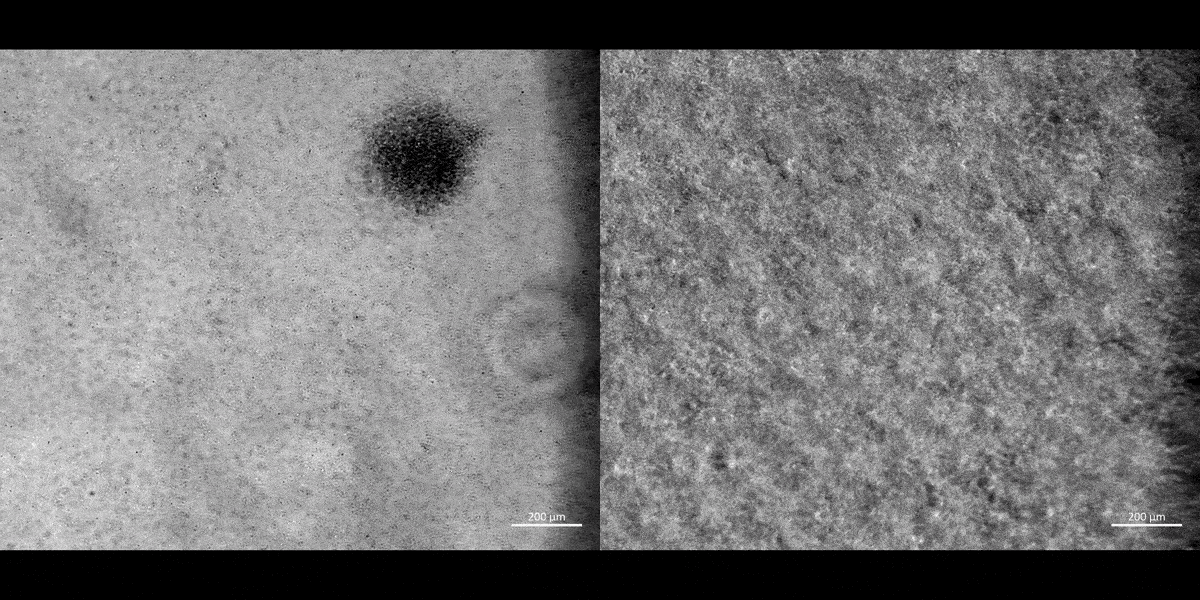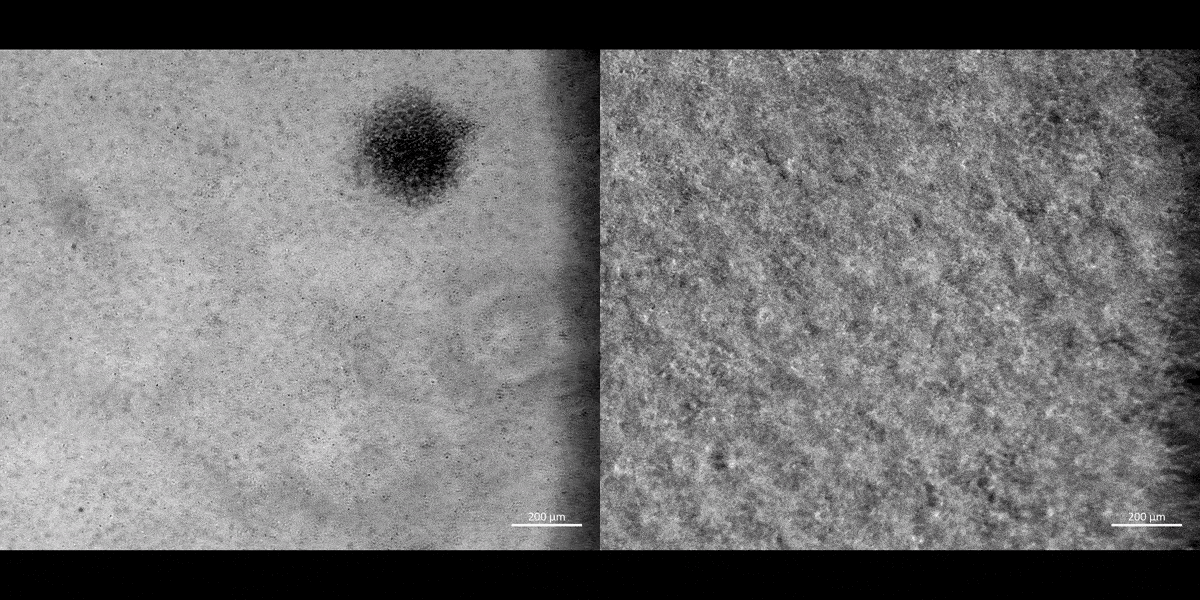
Movie 1: a) Macrophages on a chip/plate (bright field imaging): the M1 differentiated cluster-forming macrophages have a miniature morphology and are actively motile, as they aggregate in clusters on a 35 mm plate (20 x magnification). b) Macrophages on a chip/plate (bright field imaging): the M1 differentiated cluster forming macrophages on a chip are comparatively highly concentrated yet well-adjusted to the material surface and proliferate on the organ-chip instrument much better than on a plate, showing their movement and affinity for growth at a higher density, without forming distinct clusters (20 x magnification, highest magnification available to retain image clarity). Image credit: Author’s own [Jeewandara 2023].
Omics data – comparing biological themes – the genetic and epigenetic machinery
The enrichment dot-plots resulting from the bulk RNA transcriptomics data provided further validation of our subsequent core experiments. The dot-plots developed via an R-package illustrated key response pathways common to both stone formers and non-stone formers [Yu 2012] obtained via the bulk RNA sequencing data, to link the expressed genes across the analyzed patient population (Figure 10). We obtained details of mammalian gene ontology via the Jackson Laboratory GO browser. The key points showed the expression of phagosome related genes (such as FMNL1, NOD2, RAB31, SLAMF1) and phagocytosis-promoting receptors to validate our first-in-study research focus of differentiating the M1 and M2 macrophages (professional phagocytes) for functional assays on a kidney-on-a-chip instrument.
Other pathways such as the immunoglobin-like fold correspond to genes associated with cytokine-mediated signaling (CCL19, CCL21, CCR7, CCR1, CCR4). Immunoglobin isotypes related genes are involved in triggering the FcγR-expressing cells for phagocytosis, or antibody-mediated cytotoxicity in cells to activate complement pathways and deliver an immune response by activating genes such as FCGR2b, CD37, NIPAL3, SAMHD1 [Vidarsson 2014] – immune biomarkers were seen in-lab with macrophage activation during renal calcification, and identified as amyloid immune deposits with Hematoxylin and Eosin histology sections presented in our study [Jeewandara 2023, Sarioglu 2011]. Both Pleckstrin-homology domains that participate in cell signaling and cytoskeletal organization in patients with leukemia, and the leishmaniasis associated genes correspond to ‘non-stone former’ cohorts in our study. The presence of Staphylococcus aureus infection genes in the omics outcomes strengthened the concept of the association of frequent urinary tract infections with struvite stone formation during renal stone disease in patients [Jan 2008, Torricelli 2020].
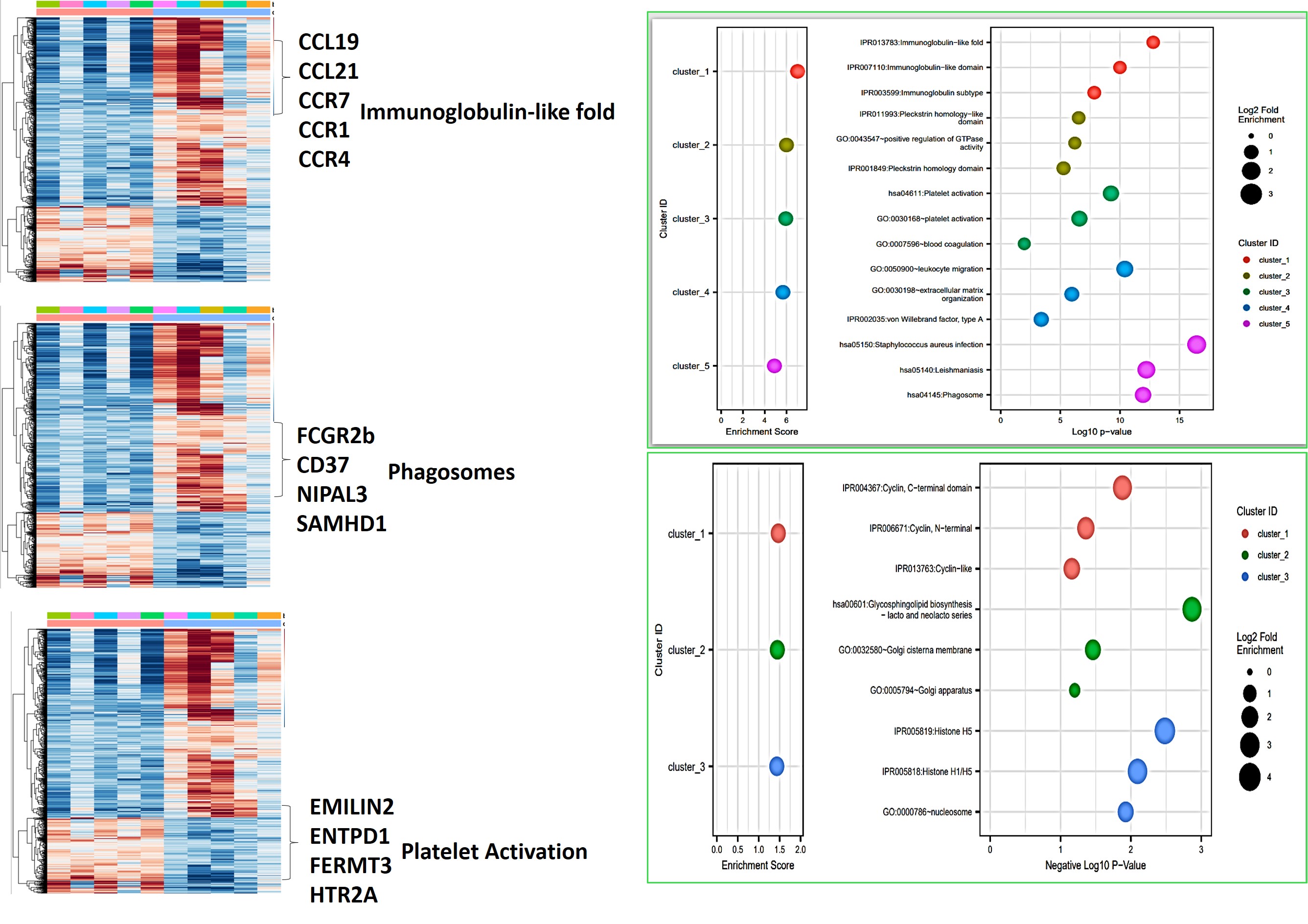
Figure 11: Bioinformatics analysis (L->R) – cluster annotation of the heatmap corresponding to genes of interest suited for gene ontology classifications, and representative dot-plot enrichment assays resulting from gene clusters of interest derived from the heatmaps. Image credit: [Jeewandara 2023].
The observed platelet activation gene cohort (EMILIN2, ENTPD1, FERMT3, ITGB3, HTR2A) is central to homeostasis and thrombosis, also associated with renal injury and epithelial endothelial dysfunction, to validate our histopathology fibrosis outcomes, eventually leading to ischemia and hypoxia in vivo; hallmarks of renal calcification [Gomchock 2023] (Figure 11). Genes related to leukocyte migration were also highlighted, including those involved in cellular extravasation and chemokine ligands associated with an inflammatory response (such as AIF1, CMKLR1, TLR8, NOD2, SPHK1). The extracellular matrix organization path contains a large cohort of genes (ADAMTS7, CCDCN80, COL1A1, MMP16, TGFB1) related to cell-matrix and collagen deposition during renal fibrosis, as I previously discussed in depth, with associations with the actin cytoskeleton cascade linked to fibrosis.
Very briefly, additional dot-plots of our study indicated cyclin-like, cyclin c-terminal, and cyclin n-terminal domains to regulate the cell cycle with pathological associations in renal carcinoma of the ‘non-stone former’ patient classifications. Histone deacetylases from the omics data play an important role in the epigenetic machinery to regulate transcriptional processes that lead to proliferation, inflammation, and fibrosis pathologies, seen with a variety of diseases and acute kidney injury, with scope to develop histone deacetylase inhibitors to treat acute kidney injury associated with renal calcification [Hyndman 2020]. Additionally, we noted the upregulation of zinc finger protein related genes (ZNF219, ZFYVE19, ZFP367, ZFP326) at the onset of pathological biomineralization via omics and energy dispersive X-ray spectroscopy data (showing an increased Zinc count), and their inhibition during cell death and calcification.
The combined dot-plot enrichment assays of our transcriptome data (Table 2), provides a gene-related precision health overview of the pathology underlying stone former vs. non-stone formers, to further justify our in vitro functional assays conducted to bottom-up engineer a pathological mechanism on a microfluidic chip [Jeewandara 2023]. To fully appreciate the broader spectrum of our transcriptomics-derived genes regulating pathological biomineralization warrants a stand-alone article.
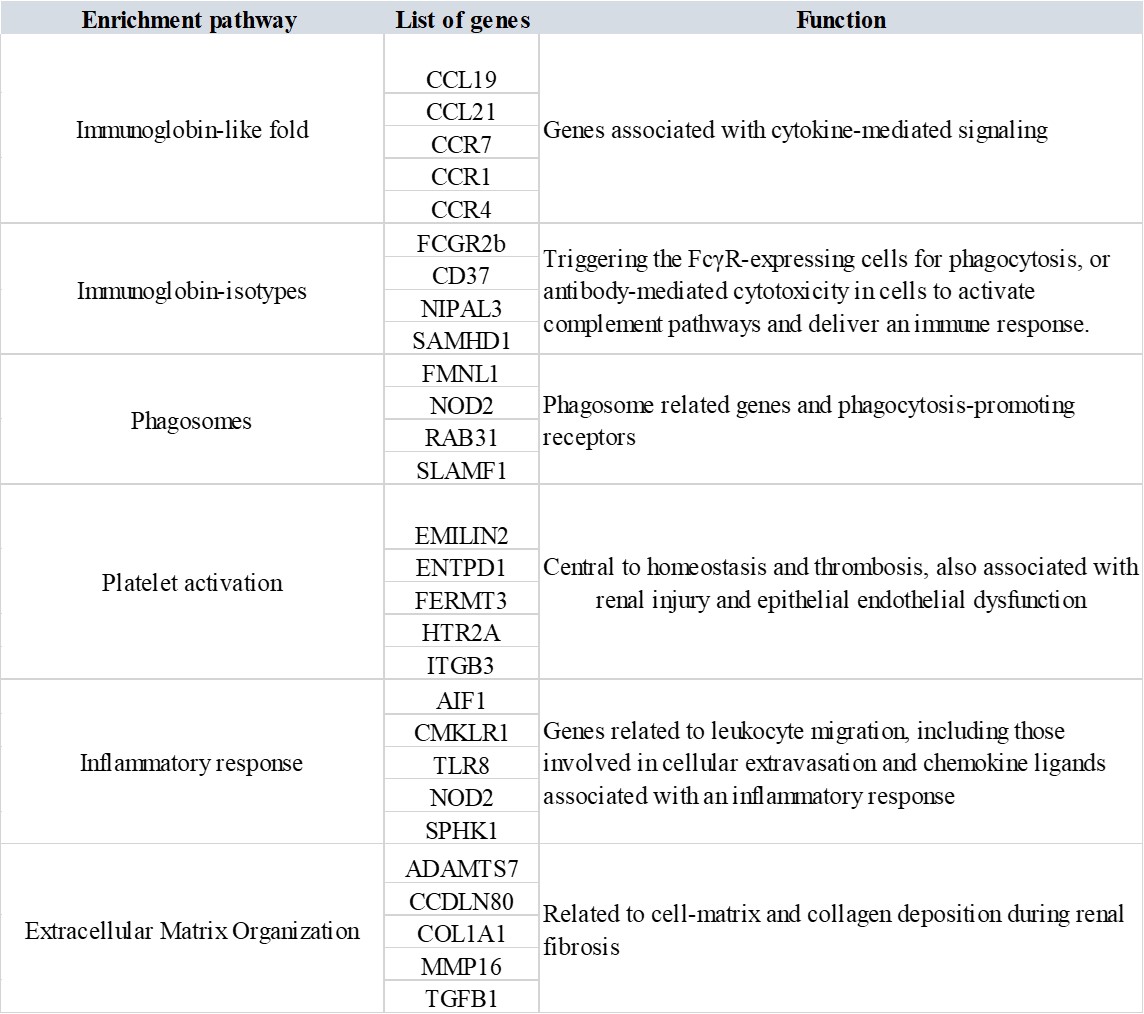
Table 2: The enrichment dot-plot derived comparisons of biological themes among gene clusters obtained via a gene ontology database, to interpret bulk RNA-sequencing-derived transcriptomics data. The analysis included all patients in the study cohort [Jeewandara 2023].
Bringing together fluorescence in-situ hybridization (FISH) and mechano-active ion receptor stress-initiated pathological mechanisms
Besides transcriptomics, we conducted fluorescence in situ hybridization (FISH) studies to identify a select set of genes, including biomarkers of inflammation by sectioning patient-derived medullapapillary complexes (Figure 12). And quantified FISH in the proximal papillae and distal papillary tip regions of both stone formers vs. non-stone former patients. The outcomes showed the differential expression of transcripts between the proximal papillae and tip. While specific genes such as GALNT3, PLEKHO1, SLCO2A1 and VCAM1 were highly expressed in stone formers at the proximal papillae, although not at the tip. The gene IGF1 and TMEM30B were highly expressed at the renal papillary tip, but not at the proximal papillae. Comparatively, the LOX transcript was highly expressed at the tip and the proximal papillae (Figure 12) [Manuscript in development].
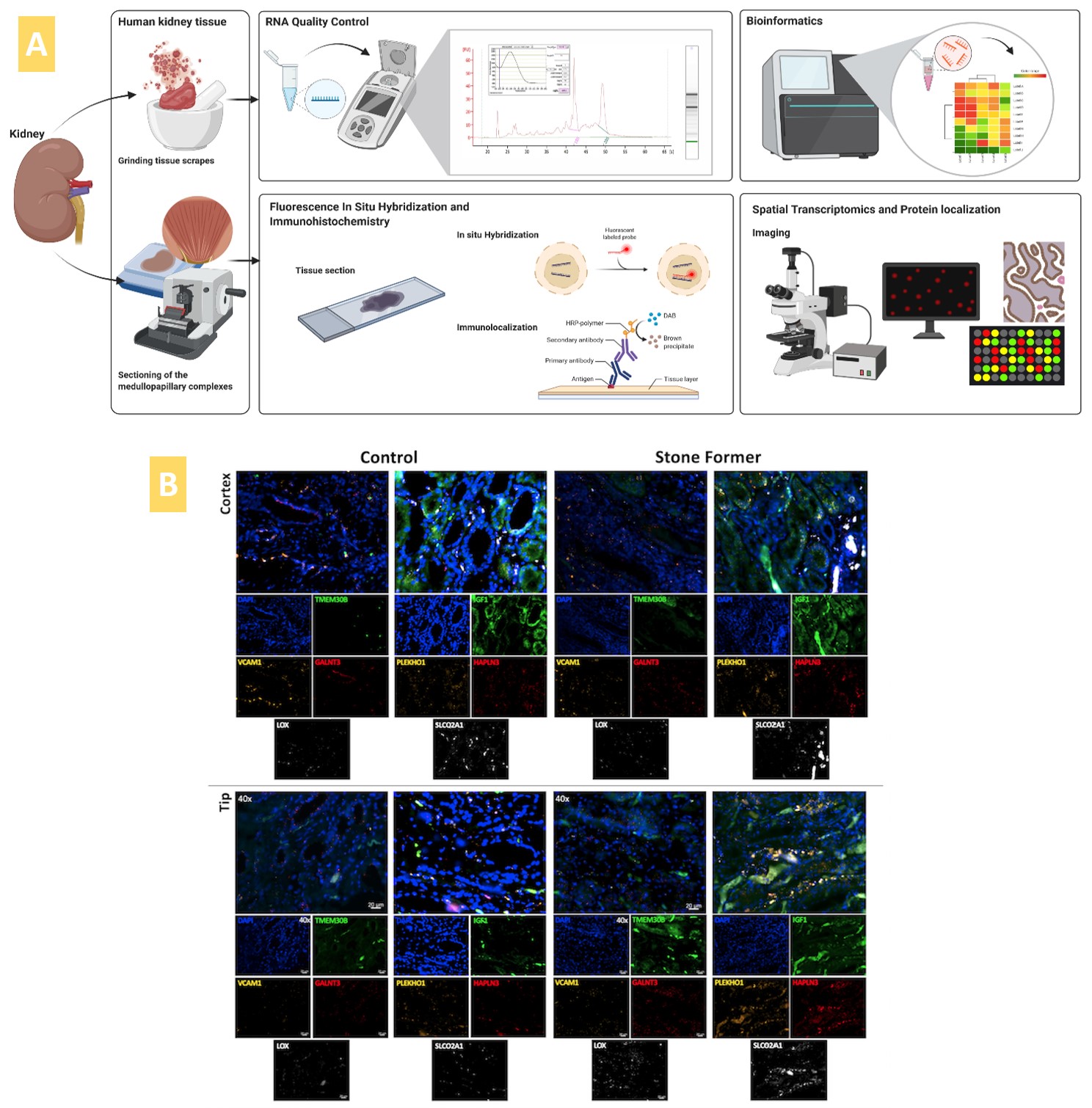
Figure 12: A) Methods of bulk RNA sequencing and FISH conducted in-lab, B) alongside FISH outcomes. Delineating the expression of key genes of interest (VCAM1, GALNT3, PLEKHO1, HAPLN3 and TMEM30B) in the renal papillary cortex and tip region of the control tissue specimen vs. stone formers. Image credit: [Manuscript in development].
Of the FISH outcomes, the endothelial cell adhesion molecule – vascular adhesion molecule1 (VCAM1) is of particular interest as a key mediator of endothelial dysfunction, induced via several factors such as reactive oxygen species, cytokines, and turbulent shear stress, during inflammation [Cook-Mills 2011]. This observation reiterates my recent views of the influence of shear-stress on activating mechanosensory ion channels such as Piezo1 and TRPV4, associated with reactive oxygen species to cause endothelial dysfunction and renal fibrosis; precursors of renal stone formation [Diaz-Ricart 2020].
These genotypes also corroborate our transcriptomics-related cascade findings of upregulated oxidative stress pathways, cytokines induced via the transforming growth factor β1 (TGFβ1) pathway, regulation of actin cytoskeleton associated with fibrosis, and p38 MAPK pathway upregulated in response to stress signals from the tissue microenvironment during kidney fibrosis and renal stone formation (Figures 8 and 10) [Jeewandara 2023]. Measuring the biomarkers of endothelial dysfunction on a kidney-on-a-chip offers a robust glimpse at precision health, as it is a key clinical feature of urolithiasis and nephrolithiasis. Endothelial function is typically assessed in the clinic by measuring flow-mediated dilation (Celermajer method) [Saenz-Medina 2021] and therapeutically attenuated with antioxidants [Cook-Mills 2011].
Mapping the bigger picture – Oxidative stress pathway and HIF1a-PHD2 pathway on a chip
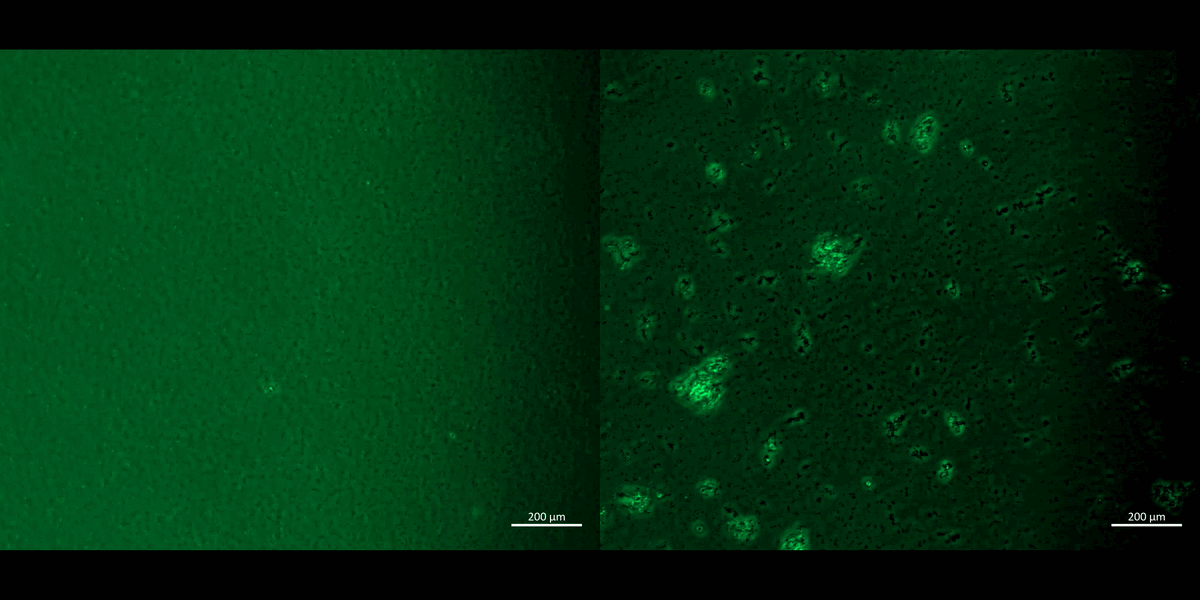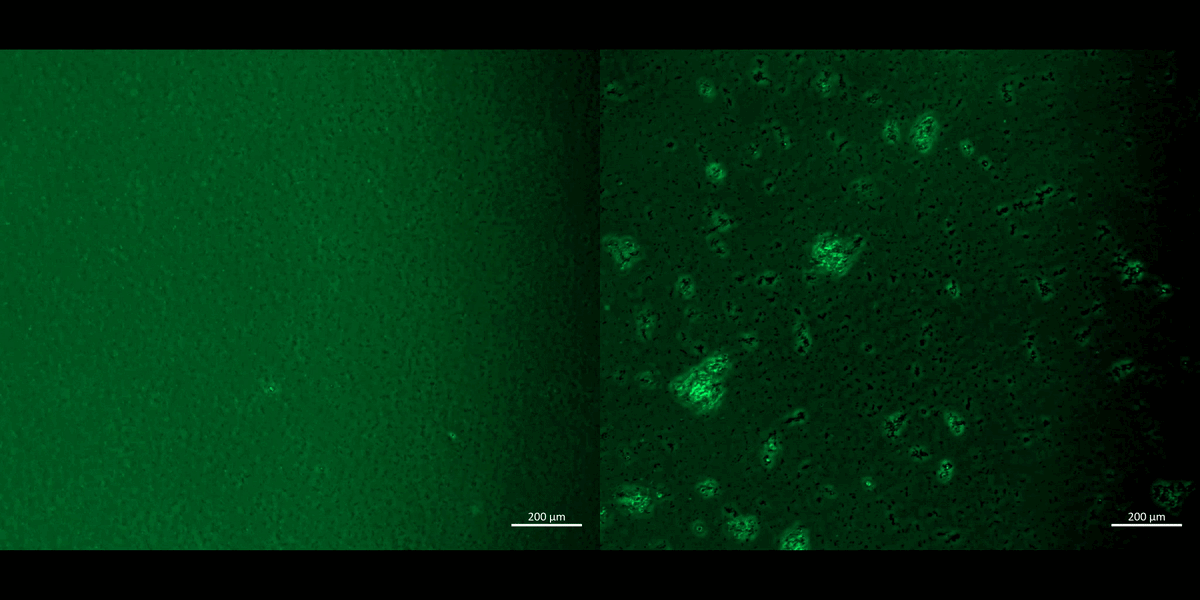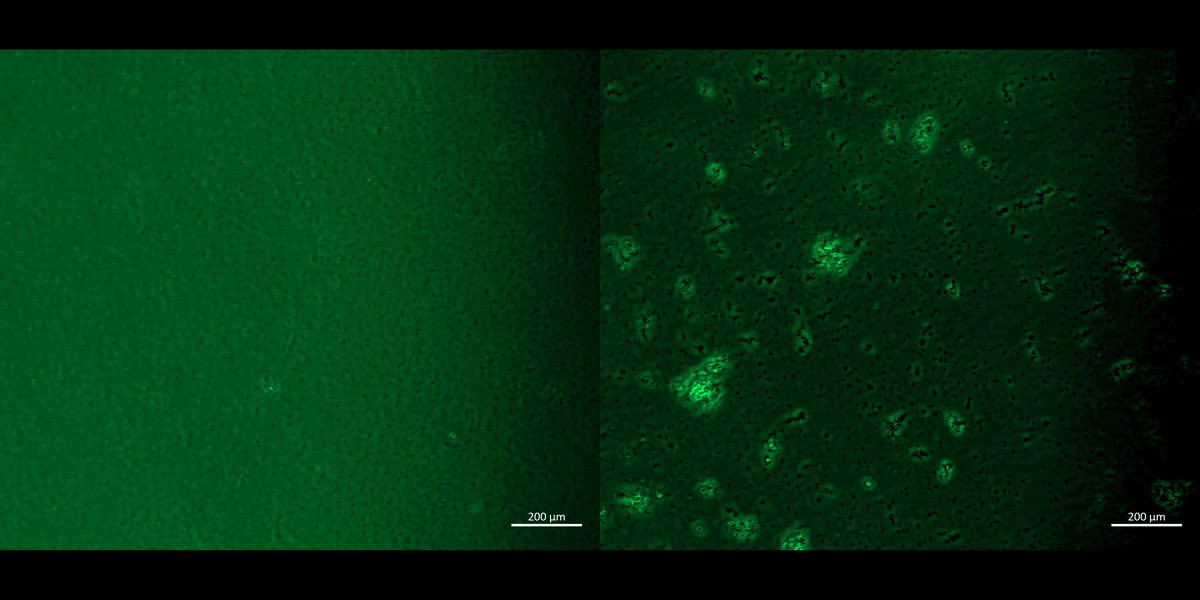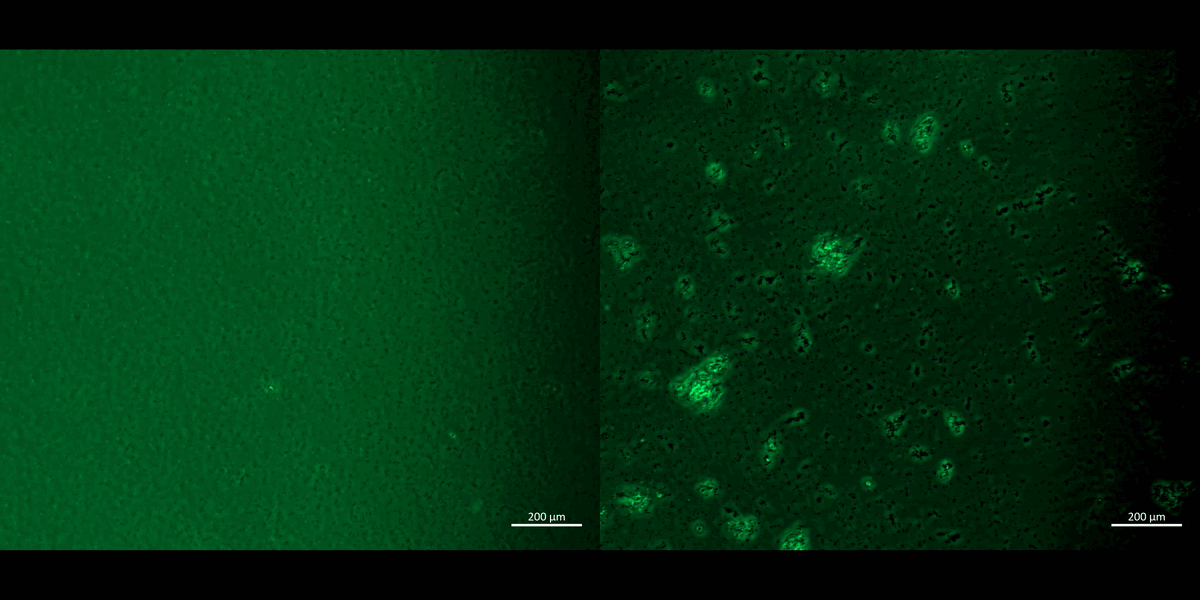
We incorporated biological ontologies/gene ontology data using the PantherDB platform to annotate genes to biological processes and observed the oxidative stress pathway and hypoxia response via the hypoxia inducible factor 1α, as two key cascades of interest during renal calcification (Figure 13), alongside pathways of chemokine signaling and cell cytotoxicity associated with cell apoptosis and cell death in nephrolithiasis.
The transcriptomics outcomes and our pathological pathways of interest corroborated our first-in-study functional assays of inducing hypoxia on a chip. We carried out the experiments by chemically inducing ischemia on M1 and M2 macrophage cell cultures in the presence of sodium dithionite and oxygen glucose deprived buffer, to facilitate oxidative stress on a chip, and observed the expression of redox biomarkers (Figure 13, Movie 2) [Jeewandara 2023]. As discussed in depth, hypoxia response via the hypoxia inducible factor 1α (HIF1α) activation is another key pathway often upregulated in the transcriptome of stone-forming patients to provide a core mechanism of HIF1α stabilization under hypoxia, to drive renal calcification (Figure 13) [Saenz-Medina 2022].
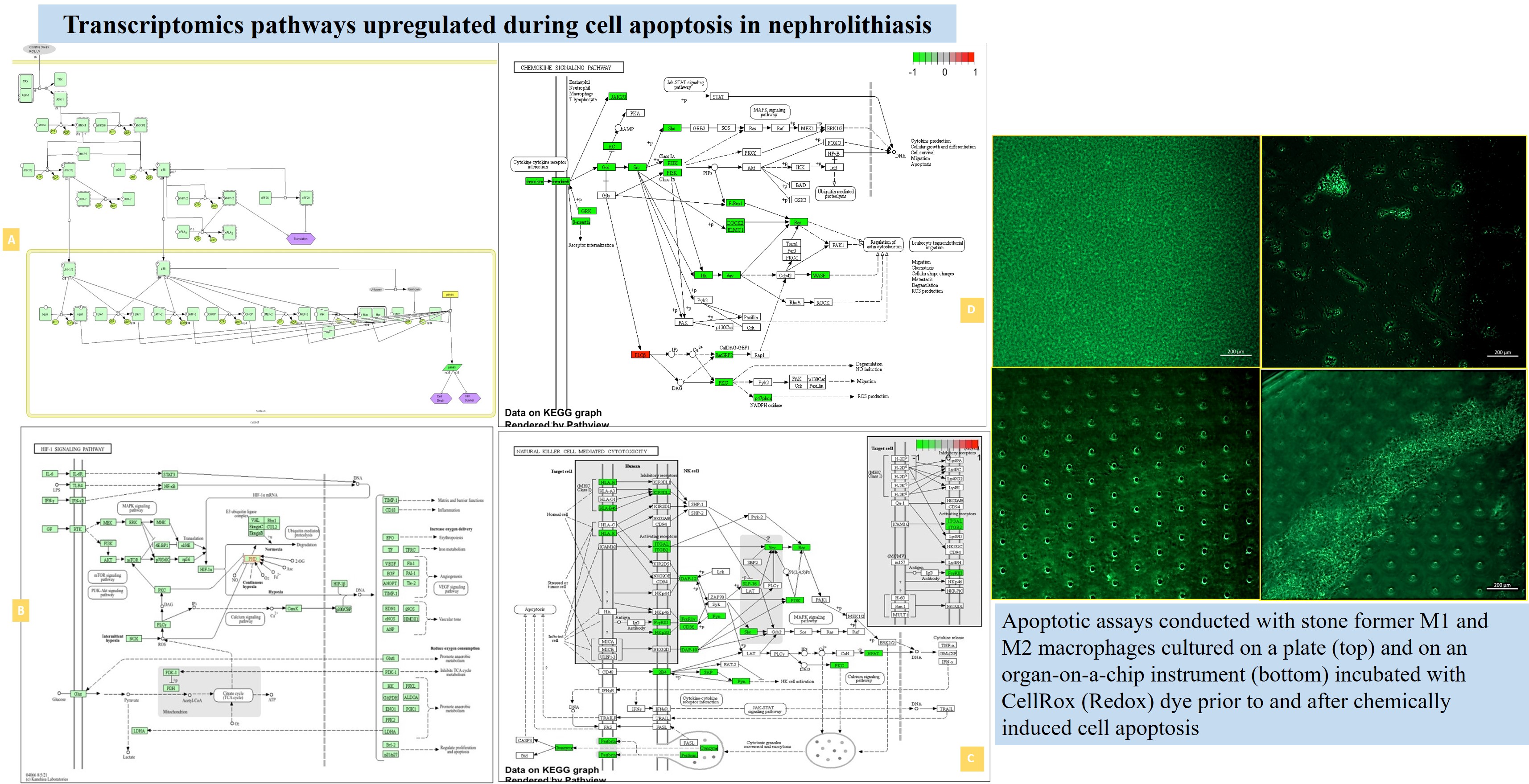
Figure 13: The KEGG meta-data and genomes of four key pathological cascades, including the oxidative stress pathway, hypoxia response via hypoxia inducible factor1α, chemokine signaling, and cell cytotoxicity linked to cell apoptosis during nephrolithiasis, obtained via the GO databases. The omics data support our functional assays of chemically induced apoptosis of M1 and M2 on a chip and a plate, incubated with a redox dye (CellRox), to show the uptake of the oxidative antibody during assays of apoptosis driven cell death (20 x magnification). Image credit: [Jeewandara 2023].
Movie 2: Apoptosis assays on a chip/plate (fluorescence imaging): the M1 and M2 macrophage cell types were incubated with CellRox redox dye and subjected to chemical ischemia-induced redox, to observe the transition from healthy motile cells (left) to fluorescent cell clumps or aggregates (right), to indicate apoptosis or cell death under ischemia, on a 35 mm tissue culture plate. The fluorescent redox dye emission (AG520) uptake indicated oxidative stress driven cell apoptosis (20 x magnification). Image credit: Author’s own [Jeewandara 2023].
Our core theme of the study further aimed to show the expression/activation of a biological switch or oxygen sensor zinc finger protein enzyme known as prolyl-hydroxylase domain 2 (PHD2) that upregulates under hypoxia at the renal papillary tip, to regulate the expression of HIF1α [Jeewandara 2023]. To delineate the HIF1a-PHD2 pathway on a chip, we emulated renal pathology on an organ chip instrument for the first time, by co-culturing patient whole blood derived, peripheral blood mononuclear cell-based macrophage types M1 and M2 differentiated under appropriate incubations in a cytokine mix (Figure 9). When these cells were incubated with the PHD2 antibody (Alexa Fluor 555) and subjected to chemically induced ischemia or hypoxia, we noticed the fluorescence signal-based activation of the PHD2 enzyme as an oxygen sensor or a switch in the cell aggregates undergoing hypoxia driven cell apoptosis (Movie 3).
The upregulation of a Zinc finger protein enzyme as an oxygen sensor at the onset of calcification coincides with our transcriptomics data (expression of ZNF proteins) in tissue samples of patients. Since chronic hypoxia response and an apoptosis-driven oxidative stress signal underly the progression of several renal stone forming pathways [Saenz-Medina 2022]. Our experimental capacity to reveal a switch or regulator of pathological oxidative stress for eventual therapeutic intervention is a significant observation with scope for therapeutic applications in the clinical treatment of nephrolithiasis and as a precision health directive for stone formers.
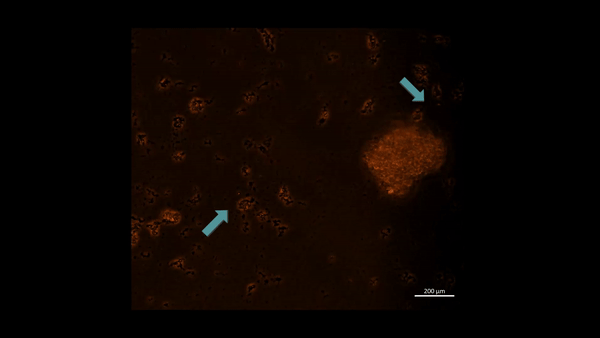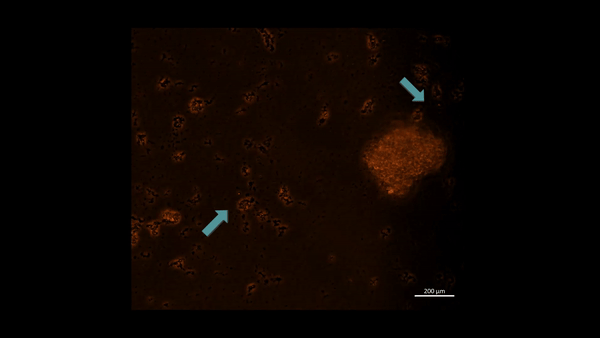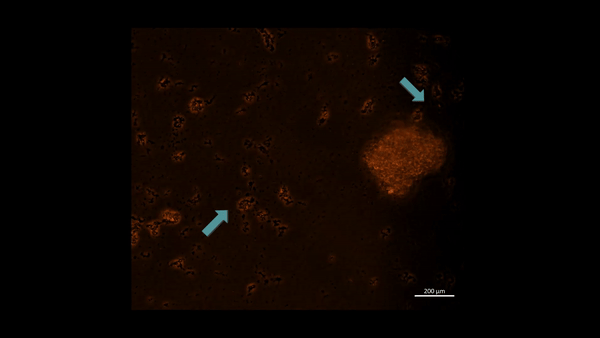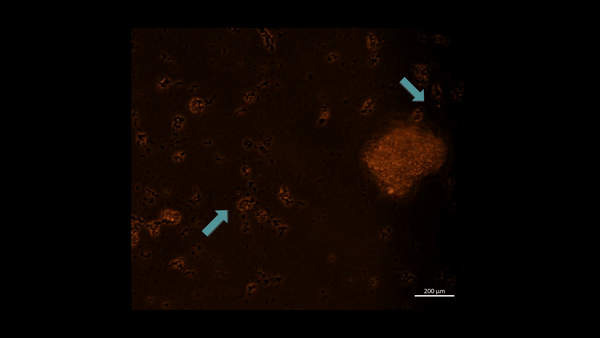
Movie 3: Hypoxia-on-a-chip/plate (fluorescence imaging): the cell types were incubated with PHD2 (Alexa Fluor 555 fluorescence tag). Upon subjecting M2 macrophage cells to chemically induced ischemia, they underwent oxidative stress and aggregated in immunofluorescent PHD2 expressing lumps, the activated PHD2 oxygen sensor enzyme within cells is noted as an oxidative stress signal that flickers on/off, as indicated with arrows (zoom in, magnification 20 x). Image credit: Author’s own [Jeewandara 2023].
Mapping the bigger picture –Epigenomics in the clinical treatment of nephrolithiasis.
Our first-in-study outcomes of omics, histopathology, and organ-on-a-chip experiments have thus far informed the development of a precision health platform on a microfluidic device to study renal stone-forming pathology, using cells derived from patients. We have addressed the key question of our study – ‘is there a biological switch at the renal tip that triggers the onset of calcification,’ with several experiments that substantiated the presence and the activation of a biological switch/oxygen sensor PHD2, to dysregulate HIF1α and attenuate hypoxia-driven calcification.
The puzzle of how cells sense and adapt to oxygen availability is an age-old question that eventually led to unveiling its vital biological phenomenon with pioneering research works, that resulted in a trio of scientists being awarded the Nobel prize in physiology or medicine in 2019. Oxygen sensitive enzymes and cellular machinery can come together to ‘turn off’ a major regulatory protagonist – i.e. hypoxia inducible factor proteins [Chopra 2020]. The very same protagonist is at play during hypoxia-driven renal calcification, with omics driven data and functional assays supporting our hypothesis of the presence of a biological switch that can regulate pathology at the renal papillae (Figure 14) [Jeewandara 2023, Arsenault 2016].
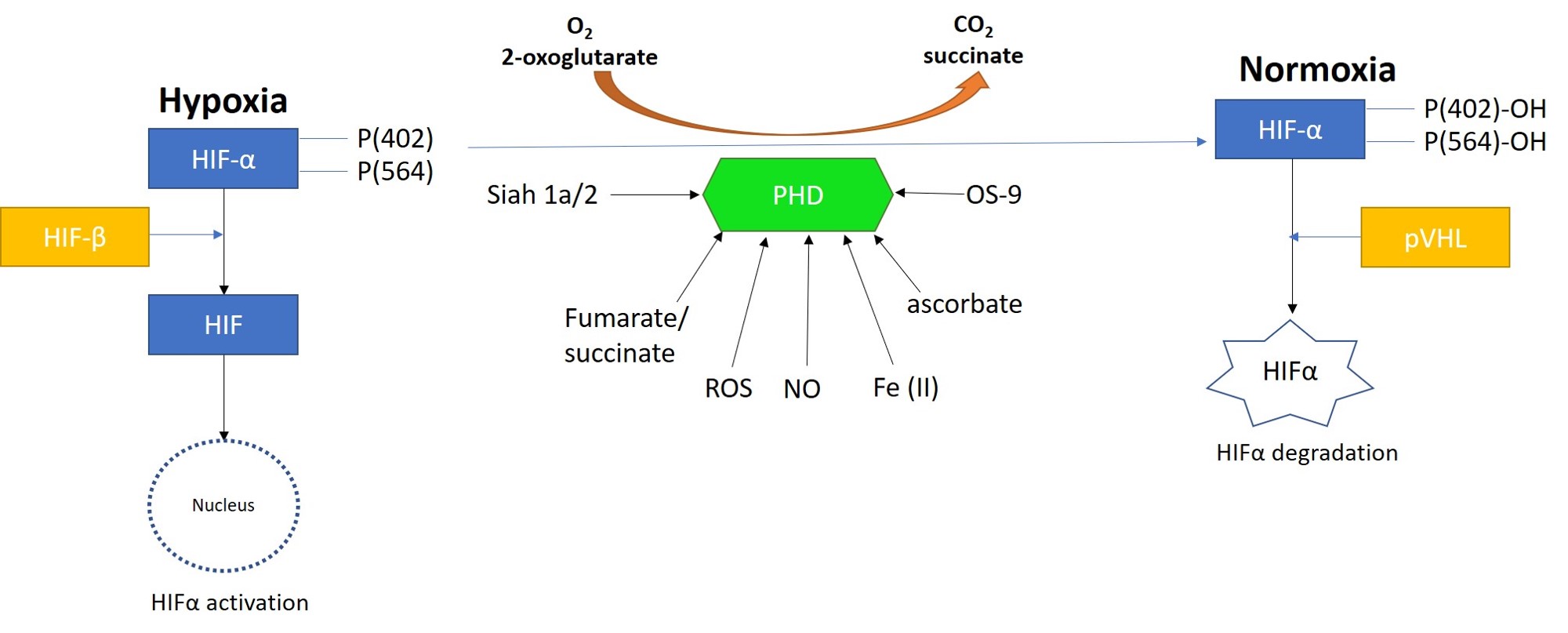
Figure 14: Prolyl hydroxylase domain 2 (PHD2) is a key oxygen sensor in mammals that post-translationally modifies hypoxia-inducible factor α (HIF1α) to target it for degradation by forming a complex with the Von Hippel-Lindau factor (pVHL). Reactive oxygen species, nitrous oxide, osteosarcoma amplified 9, ascorbate, succinate, iron, and protein ligase components can regulate the function of PHDs. Image credit: Author’s own.
Mapping the bigger picture - Investigating molecular medicine and epigenetic drugs
So how do we integrate these outcomes on a precision health platform, to design and develop a small molecule drug candidate that can treat chronic oxidative stress driven hypoxia, at the onset of renal stone formation?
The capacity to explore the mechanism-of-action of epigenetics drugs in complex diseases is a niche that I previously wrote about, with direct implications for personalized therapy in renal stone disease. We can explore the epigenetic cellular machinery underlying the HIF1α-driven oxidative stress pathway to integrate these outcomes on a precision health platform. During chronic hypoxia, for instance, when oxygen-sensor molecules such as PHD2 are inhibited, lysine methyltransferase enzymes G9a and G9a-like proteins (GLP) are upregulated to directly bind the alpha subunit of HIF1α and induce methylation for continued inhibition of the hypoxic protein [Chopra 2020]. DNA methylation mediated via the G9a, and G9a-like proteins can suppress the HIF1α transcription activity, to inhibit HIF1α by reducing its transactivation domain function [Shinkai 2011].
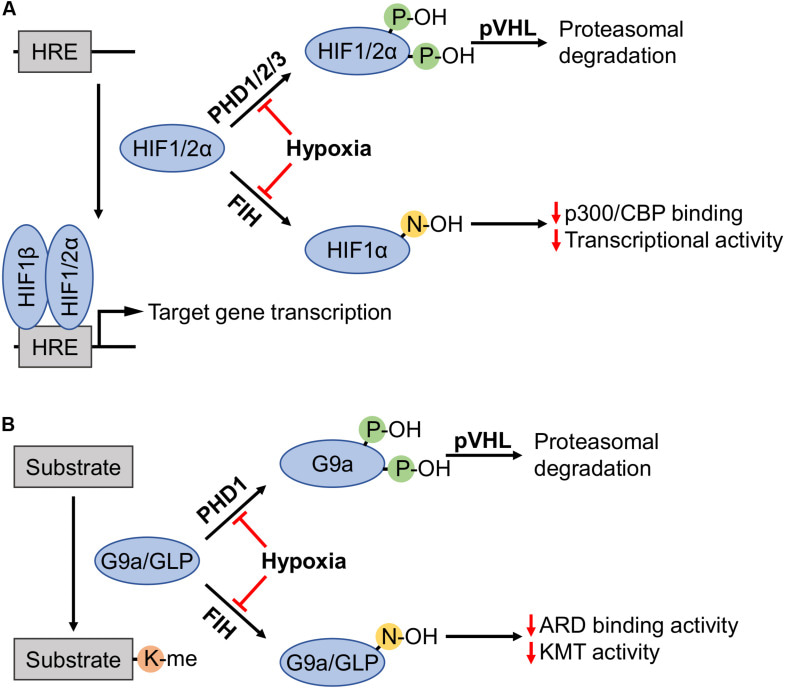
Figure 15: Highlighting the proteasomal degradation pathway and the regulation of HIF1α, G9a and GLP by the oxygen sensor prolyl hydroxylase domain 2 enzyme (PHD2) under normoxia. (A) Prolyl hydroxylases (PHDs) in the presence of sufficient oxygen levels (normoxia) induce the recognition and degradation of HIF1α via von Hippel Lindau tumor suppressor protein (pVHL). Factor inhibiting hypoxia has a similar role. (B) At the onset of hypoxia, HIF1α undergoes methylation induced via G9a and GLP for its suppression in an alternate pathway of HIF1α degradation [Chopra 2020].
This is an alternate pathway of HIF1α degradation when compared to the PHD2-regulated pathway of Von Hippel Lindau protein binding and ubiquitination of HIF1α (Figures 14 and 15) [Arsenault 2016]. This alternate path highlights the epigenetic role of G9a to be larger than what is currently known, and its existing scope as a druggable target to design personalized molecular medicine and regulate pathological hypoxia in patients predisposed to chronic renal calcification. At normoxia, the oxygen sensor PHD2 can suppress all factors including HIF1α, and the hypoxia-inducible G9a, and GLP proteins to attenuate oxidative stress related factors, and reach equilibrium. The methylation-driven epigenetic mechanism can be investigated during oxidative stress-related renal biomineralization, with cross-disciplinary potential to also regulate oncogenesis [Chopra 2020].
Wrapping-it-up - integrating study outcomes on a precision health platform
Our comprehensive study of nephrolithiasis that investigates a pathological cascade of renal calcification by bottom-up engineering pathological biomarkers on an organ-on-a-chip instrument, can inform precision health on a chip, by bringing together a range of interdisciplinary themes of transcriptomics and histology. The core study outcomes, listed below, were established by using clinical samples of stone formers vs. non-stone formers to guide the development of functional assays on a chip. The study outcomes can guide the implementation of precision health on a kidney-on-a-chip for personalized clinical translation (Figure 16), based on the following factors:
- We present a robust microfluidic platform to recreate patient pathology for precision health, to facilitate precision diagnostics and precision medicine at a personalized level.
- We successfully differentiated, established, and validated macrophages on a kidney-on-a-chip platform to explore biomarkers of renal calcification using M1 and M2 macrophages developed in-lab for the first time.
- We identified the prolyl-hydroxylase domain 2 (PHD2) protein, a key biomolecule that acts as a biological switch to regulate hypoxia inducible factor 1α (HIF1α), by emulating hypoxia-driven oxidative stress on a kidney-on-a-chip – with scope for therapeutic intervention.
- Epigenetics mechanisms underlying pathological biomineralization can be investigated on a microfluidic platform, to gain new insights to clinically attenuate the pathways of interest.
- Further in-lab studies with macrophage cell cultures can explore additional omics guided inflammatory cascades that are upregulated during stone formation such as the p38 MAPK pathway, to therapeutically regulate the expression of M2 anti-inflammatory macrophages to attenuate renal calcification in tissues with birefringent calcium oxalate sediments.
- Immunohistochemistry studies can be conducted with the PHD2 antibody to identify its expression on renal tissue samples exhibiting nephrolithiasis.
- Drug-pathway associations can be developed on a chip, by integrating top-down modeling methods of machine learning to plan and facilitate personalized medicine platforms.
- This capacity to provide patients with a personalized view of their pathology recreated on a chip to optimize disease diagnosis, and treatment strategies can revolutionize healthcare.
In this way, we have designed and developed a roadmap for personalized healthcare, supported by patients’ transcriptomics, and histology data to introduce customized, low-cost precision therapeutics platforms on an organ-on-a-chip platform, preceded with functional assays to establish biomarkers of renal calcification. These state-of-the-art microphysiological instruments have scope to understand and treat renal calcification and other complex diseases.
Header Image: an artistic rendition of a region of the renal cortex of stone-formers with glomeruli stained with Masson's Trichrome to highlight biomarkers of collagen, and tissue pathology. Image credit: Author's own.
References
- Editorial., What will it take to make precision health a global reality, Nature Medicine, 2024
- Duffield J. et al. Cellular and molecular mechanisms in kidney fibrosis, The Journal of Clinical Investigation, 2014.
- Rose J. et al. Comprehensive proteomic quantification of bladder stone progression in a cystinuric mouse model using data-independent acquisitions, PLoS One, 2022.
- Coe F. et al. Three pathways for human kidney stone formation, SpringerLink, 2010.
- Saenz-Medina J. et al. Endothelial Dysfunction: An Intermediate Clinical Feature between Urolithiasis and Cardiovascular Diseases, MDPI 2022
- Jeewandara T. et al. The dynamics of pathological biomineralization in the renal papillae, World Congress of Nephrology 2023, abstract number: WCN23:0323, 2023.
- Arsenault P. et al. The Zinc Finger of Prolyl Hydroxylase Domain Protein 2 Is Essential for Efficient Hydroxylation of Hypoxia-Inducible Factor α, Molecular and Cellular Biology, 2016.
- Misra S. Precision health could mitigate clinical biases that impact care, Nature Medicine, 2024
- Azer K. et al. Systems biology platform for efficient development and translation of multitargeted therapeutics, Frontiers, 2023
- Wang C. et al. Drug-pathway association prediction: from experimental results to computational models, Briefings in Bioinformatics, 2020.
- Ho S. et al. Architecture-Guided Fluid Flow Directs Renal Biomineralization, Scientific Reports, 2018.
- Dugger S. et al. Drug development in the era of precision medicine, Nature Reviews Drug Discovery, 2017.
- Hartung T. et al. Toxicology for the 21st Nature, 2009.
- Ashammakhi N. et al. Kidney-on-a-chip: untapped opportunities, Kidney International, 2018.
- Jang K. et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment, Integrated Biology, 2013.
- Rhee E. How Omics Data Can Be Used in Nephrology, American Journal of Kidney Diseases, 2019.
- Yu G. et al. ClusterProfiler: an R Package for Comparing Biological Themes Among Gene Clusters, 2012.
- Ashburner M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium, Nature Genetics, 2000.
- Yang Y. et al. Functional Roles of p38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses, Mediators of Inflammation, 2014.
- Khan S. et al. Randall's plaque and calcium oxalate stone formation: role for immunity and inflammation, Nature Reviews Nephrology, 2021.
- Mosser D. et al. Exploring the full spectrum of macrophage activation, Nature Reviews Immunology, 2008.
- Yang H. AUA2023 BEST POSTERS Tissue Resident Macrophages Are Associated With Randall’s Plaques, American Urological Association, 2023.
- Zhou D. et al. Sonic hedgehog signaling in kidney fibrosis: a master communicator, SpringerLink, 2017
- Gao J. et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function, Cell Stem Cell, 2010.
- Ushach I. et al. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage, Journal of Leukocyte Biology, 2016.
- Taguchi K. et al. Macrophage function in calcium oxalate kidney stone formation: A systematic review of literature, Frontiers Immunology, 2021.
- Vidarsson G. et al. IgG Subclasses and Allotypes: From Structure to Effector Functions, Frontiers Immunology, 2014.
- Sarioglu S. et al. Quantification of immune deposits in renal diseases, Applied Immunohistochemistry and Molecular Morphology, 2011.
- Jan H. et al. Frequency of renal stone disease in patients with urinary tract infection, Journal of Ayub Medical College, 2008
- Torricelli F. et al. Staghorn renal stones: what the urologist needs to know, 2020
- Gomchock D. et al. Platelets in Renal Disease, MDPI, 2023
- Hyndman K. Histone Deacetylases in Kidney Physiology and Acute Kidney Injury, Seminars in Nephrology, 2020
- Cook-Mills J. et al. Vascular Cell Adhesion Molecule-1 Expression and Signaling During Disease: Regulation by Reactive Oxygen Species and Antioxidants, Antioxidants and Redox Signaling, 2011.
- Diaz-Ricart M. et al. Endothelial Damage, Inflammation, and Immunity in Chronic Kidney Disease, Toxins (Basel) 2020.
- Saenz-Medina J. et al. Urolithiasis Develops Endothelial Dysfunction as a Clinical Feature, Antioxidants (Basel), 2021.
- Chopra A. et al. Hypoxia-Inducible Lysine Methyltransferases: G9a and GLP Hypoxic Regulation, Non-histone Substrate Modification, and Pathological Relevance, Frontiers Genetics, 2020.
- Shinkai Y. et al. H3K9 methyltransferase G9a and the related molecule GLP, Genes and Development, 2011.
Follow the Topic
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcement



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in