Prioritized Na+ Adsorption‑Driven Cationic Electrostatic Repulsion Enables Highly Reversible Zinc Anodes at Low Temperatures
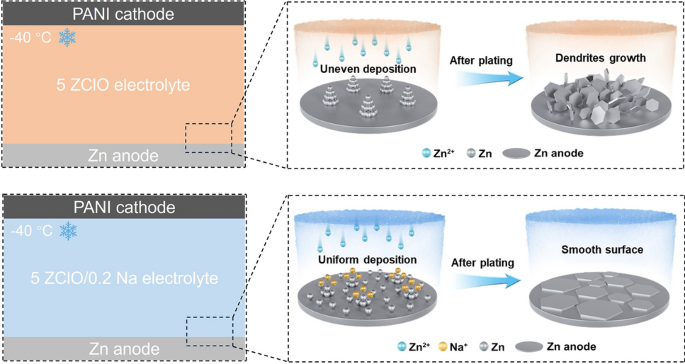
As renewable energy storage demand grows, the limitations of aqueous zinc metal batteries (AZMBs) in subzero environments become more pronounced. Now, researchers from Harbin University of Science and Technology and Fudan University, led by Professor Xin Liu and Professor Dongliang Chao, have presented a breakthrough solution using trace Na2SO4 as an electrolyte additive. This work offers valuable insights into developing next-generation energy storage technologies that can overcome low-temperature challenges.
Why Na2SO4 Matters
- Cost-Effective: Na2SO4 is an abundant, low-cost inorganic salt that avoids the flammability and conductivity issues of organic antifreeze agents
- Electrostatic Regulation: Na+ preferentially adsorbs at the zinc anode surface, repelling Zn2+ and promoting uniform deposition
- Suppressed Side Reactions: Fewer water molecules coordinate with Na+, reducing hydrogen evolution and harmful byproducts
- Ultra-Stable Cycling: Zn||Zn cells achieve over 2500 hours at −40°C, and Zn||PANI full cells retain >90% capacity after 8000 cycles
Innovative Design and Features
- Electrolyte Formulation: The optimized 5ZClO/0.2Na electrolyte (5m Zn(ClO4)2+ 0.2m Na2SO4) maintains high ionic conductivity down to −60°C
- Hydrogen Bond Disruption: Spectroscopic analyses reveal Na2SO4 further breaks water-water hydrogen bonds, enhancing antifreeze capability
- Interfacial Engineering: DFT calculations show Na+ adsorption energy (−7.32 eV) exceeds Zn2+(−2.49 eV), enabling preferential interface regulation
- Nucleation Control: Na+ increases nucleation overpotential, promoting denser, more uniform zinc deposition
Applications and Future Outlook
- Low-Temperature Energy Storage: Enables reliable battery operation in extreme cold environments for EVs and grid systems
- Long-Life Power Supplies: Zn||Cu cells maintain 99.5% Coulombic efficiency for >1000 cycles at −40°C
- Safe and Scalable: Organic-free design maintains aqueous battery safety while improving performance
- Challenges and Opportunities: Future work will focus on optimizing additive concentrations and exploring other cost-effective inorganic salts
This breakthrough provides a roadmap for developing high-performance, low-temperature AZMBs using simple electrolyte modifications. It highlights the importance of interfacial engineering and cost-effective materials design in advancing energy storage technologies. Stay tuned for more groundbreaking work from Professor Xin Liu and Professor Dongliang Chao's teams!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in