“Proton‑Iodine” Regulation of Protonated Polyaniline Catalyst for High‑Performance Electrolytic Zn‑I2 Batteries
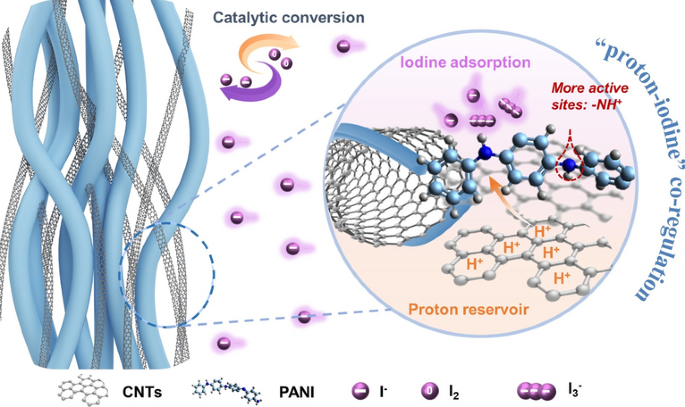
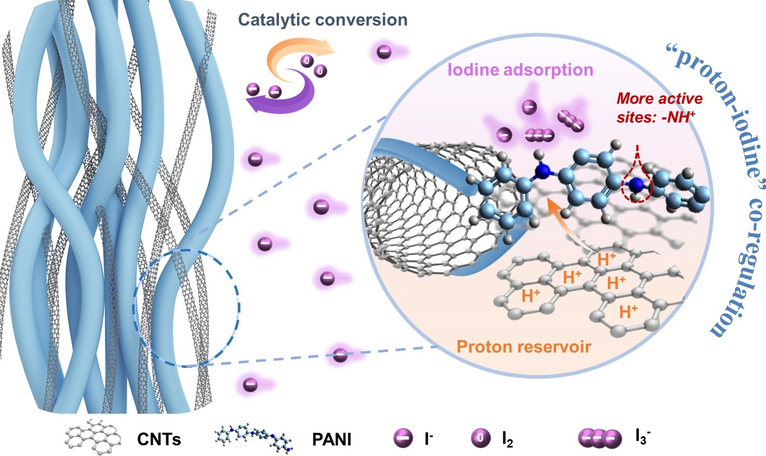
As demand for safe, low-cost and grid-scale storage keeps climbing, aqueous Zn–I2 batteries attract intense attention yet are dogged by polyiodide shuttling and sluggish iodine conversion. Now, researchers from Beijing University of Chemical Technology and Beijing Institute of Technology, led by Prof. Yi Zhao, present a catalytic cathode that finally tames these twin problems. Their three-dimensional carboxyl-carbon-nanotube-wrapped polyaniline (C-PANI) realises a direct I0/I- redox, delivering 420 mAh g-1 and an ultra-long lifespan over 40 000 cycles.
Why C-PANI Matters
- Proton Reservoir: Carboxyl-CNTs fix H+ from mild electrolyte, generating abundant –NH+–/–NH+= sites in PANI for rapid iodine anchoring.
- Shuttle Suppression: "Proton–iodine" co-regulation prevents polyiodide formation and Zn corrosion.
- Scale-up Ready: 30 g-batch powder and 60 mAh pouch cells retain 90.8 % after 100 cycles.
Innovative Design & Features
- Catalytic Framework: Solvothermal-stirred PANI nanorods entangled with carboxyl-CNTs form neuron-like conductive networks.
- Capacitive Kinetics: 79 % capacitive contribution at 1.2 mV s-1 and low Tafel slopes (73.7 mV dec-1) enable 20 A g-1 operation.
- DFT Validation: Gibbs barrier drops from 0.933 eV (PANI) to 0.869 eV (C-PANI); binding energies of I-, I2 and I3- all decrease.
Applications & Outlook
- High-energy Pouch: 72 mAh cell powers LED board, proving practical applicability.
- Green Manufacturing: Room-temperature processing avoids toxic I2 vapour.
- Next Steps: Team will optimise N/P ratio and scale tape-casting for kWh-level packs.
This work offers a scalable organocatalyst pathway to long-life, high-rate Zn–I2 batteries and provides new insight into proton-involved energy chemistry. Stay tuned for more advances from Prof. Yi Zhao’s group!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in