Reflections from the Genomics England Health Summit 2025: SDGs 3, 10 and DEI in Genomic Medicine
Published in Cancer and Genetics & Genomics

This discussion, written for the SDG 3 Newsletter August Issue: Access For All, contributes to Sustainable Development Goals 3 and 10, Good Health and Wellbeing and Reduced Inequalities, respectively.
Genomics and the Publishing Landscape, Abiola Lawal
Last month’s Genomics England Health Summit was a vibrant gathering of minds from across the genomic research and healthcare landscape. Held at the Business Design Centre in London, the event brought together researchers, clinicians, industry leaders and patient advocates to explore the transformative potential of genomics in healthcare. As Publishers of genomics research journals, India and I went along to represent BMC.
Connecting with the Community
One of the most rewarding aspects of the summit was the opportunity to meet so many passionate individuals—both familiar faces and new connections. These conversations were not only energising but also deeply informative. We received direct feedback from researchers and clinicians, particularly around the publishing process, which will help us better tailor our support for the genomics community.
Supporting Researchers More Effectively
A recurring theme in our discussions was how publishers can better support researchers in genomics. Attendees expressed a strong interest in:
- Faster and more transparent peer review: BMC’s peer review standard
- Greater visibility for interdisciplinary work
- Support for data sharing and reproducibility: see BMC's Data sharing policy and Springer Nature’s Research Data policy.
The Role of Transformative Agreements
Another hot topic was the relevance of Transformative Agreements (TAs) in shaping where authors choose to publish. Many researchers are now more aware of how these agreements can remove financial barriers to open access publishing, especially when their institutions are covered. This is a crucial step toward making genomic research more accessible and impactful.
Bridging the Gap Between Research and Patients
A powerful message echoed throughout the summit was the importance of bringing researchers closer to patients—and patients closer to research. This includes:
- Linking registries and patient data to enable more comprehensive, longitudinal insights into health and disease. (Shout out to the ISRCTN Registry!)
- Empowering patients as partners in research, not just participants.
- Recognising patient contributions through authorship, especially in co-designed studies or those involving patient-led data collection and interpretation.
- Improved access/readership for patients and families to research: These shifts are not only ethically important—they also enrich the quality and relevance of genomic research. It also highlights the importance of Open Access and makes BMC proud to be pioneers in OA publishing: we make research free to access for everyone, everywhere.
Emerging Themes for Collections
Several emerging research themes stood out as potential candidates for future Collections (also known as Special or Focus Issues). These included:
- Pharmacogenomics and personalised medicine
- Rare disease diagnostics and the Generation Study
- AI and machine learning in genomic data interpretation
- Equity and ethics in genomic healthcare
- Patient-led research and citizen science in genomics
We are excited to explore these themes further and invite researchers—and patient collaborators—to contribute to upcoming Collections that spotlight these critical areas.
SDGs 3, 10 and DEI in Genomic Medicine, India Sapsed-Foster
Genomics is becoming an integral part of mainstream healthcare and, by 2035, could contribute to addressing half of all health conditions. It is increasingly enhancing the diagnosis, treatment and prevention of rare diseases and cancer, supporting progress toward SDG 3 (Good health and Wellbeing). As the NHS becomes more digital and effective, clinicians are leveraging genomic therapies, testing and digital infrastructure to improve diagnostic accuracy. Recent advances in genomics research led to the discovery of ReNu Syndrome in 2024.
Speakers at the Genomics Research Summit highlighted the crucial role of clear and ongoing communication between scientists and participants during clinical trials and research studies. Incorporating patients’ and participants’ lived experiences, health priorities and personal preferences is essential to conducting meaningful research. Sharing study outcomes and feedback with participants not only acknowledges their contribution but also encourages future participation, fostering greater engagement and trust. Currently, participants are rarely informed on how their data has been used once research is published or studies conclude. Providing this transparency would be far more effective in reinforcing their value in the research process.
It is increasingly clear that scientists must communicate more openly about how participants' data is used, why it matters, and how the research benefits the wider community. This kind of transparency and dialogue is crucial to building lasting trust – particularly among Black, ethnic minority, and other marginalised communities, where historical injustices have led to deep-rooted mistrust in medical research (see also our previous Q&A). It is essential to rebuild trust with Black and ethnic minority communities to ensure they are better represented in health data, have equal access to healthcare, and ultimately achieve more positive health outcomes (SDG 10: Reduce inequality within and among countries).
This point was also emphasised by Dr. Irene Aninye, Chief Science Officer at the Society for Women’s Health Research, during the Organisation for the Study of Sex Differences 2025 Annual Meeting in Albuquerque, which I recently attended (see also our Q&A with Dr. Aninye and her accompanying Editorial ‘A call for inclusive research, policies, and leadership to close the global women’s health gap’ published in Biology of Sex Differences). Now is the time to decolonise the healthcare system, which remains affected by systemic racism. I highly recommend the insightful book Divided by Dr. Annabel Sowemimo, which explores this ongoing issue in depth.
Furthermore, white individuals are diagnosed with health conditions and diseases at higher rates than those from Black and ethnic minority backgrounds. This disparity is not due to genetics alone. People from minority groups are often overlooked, misdiagnosed, or not referred for appropriate care despite presenting with symptoms (SDG 10). This neglect contributes to higher mortality rates within these communities (SDG 3.b).
 For example, in his talk “DEI and Genomic Medicine,” Professor Matthew Brown highlighted a key example from 2015, when the World Health Organisation (WHO) recommended shifting from a three-drug to a single-drug HIV treatment regimen in Zimbabwe and other African countries where HIV prevalence is high (with 15–20% of adults in Zimbabwe affected). However, the drug had been tested primarily on white European populations, and only after its rollout was it discovered that the plasma concentration triggered adverse psychiatric reactions in many Zimbabwean patients. This led thousands to discontinue treatment. This case underscores one of the many reasons for the deep-rooted lack of trust in healthcare systems among underrepresented communities. In early HIV treatment studies, some participants, allegedly, pretended to take the medication but claimed otherwise to avoid disappointing researchers, resulting in inaccurate data and further erosion of trust.
For example, in his talk “DEI and Genomic Medicine,” Professor Matthew Brown highlighted a key example from 2015, when the World Health Organisation (WHO) recommended shifting from a three-drug to a single-drug HIV treatment regimen in Zimbabwe and other African countries where HIV prevalence is high (with 15–20% of adults in Zimbabwe affected). However, the drug had been tested primarily on white European populations, and only after its rollout was it discovered that the plasma concentration triggered adverse psychiatric reactions in many Zimbabwean patients. This led thousands to discontinue treatment. This case underscores one of the many reasons for the deep-rooted lack of trust in healthcare systems among underrepresented communities. In early HIV treatment studies, some participants, allegedly, pretended to take the medication but claimed otherwise to avoid disappointing researchers, resulting in inaccurate data and further erosion of trust.
Therefore, data from non-European countries remains limited, and ethnic and marginalised communities are often underrepresented in research, largely due to issues of trust, among other factors, which contributes to significant disparities in healthcare. In genomics research specifically, studies are more commonly conducted in high-income countries where infrastructure, education, funding and technology make research more accessible and feasible (SDG 3.c; 3.8). This leads to a disproportionate focus on European populations. Additionally, some European countries restrict access to their data for researchers in non-European nations, further limiting global representation in genomics research and preventing others from building on that data to benefit their own populations and ethnic groups (SDG 10).
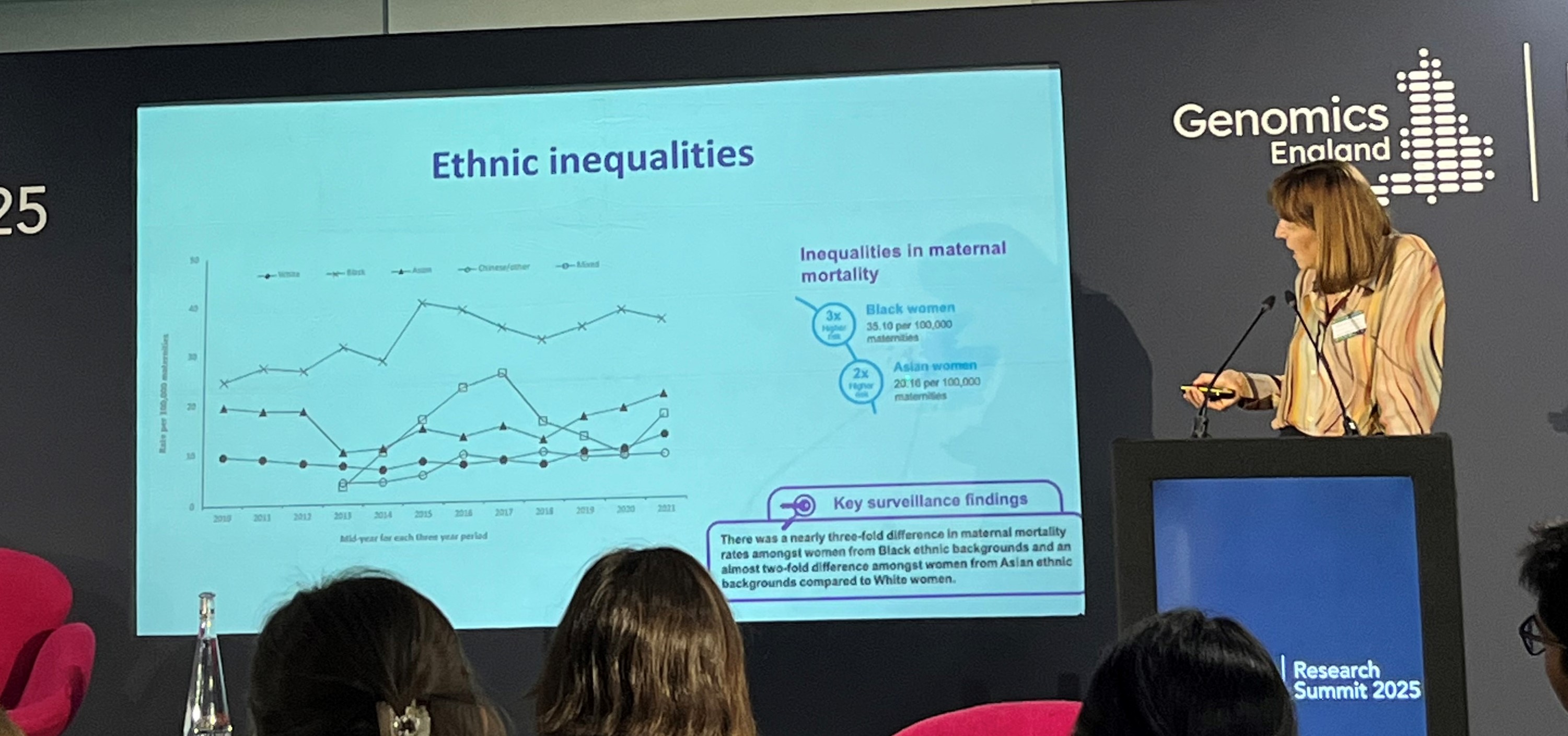 In her presentation “Pregnancy Disorders and Ethnic Diversity: Returning Results,” Professor Catherine Williamson highlighted that pregnancy disorders (such as gestational diabetes, pre-eclampsia and pre-term birth) disproportionately affect Black and ethnic minority women. She also reiterated the statistic that Black women are three times more likely to die from pregnancy-related complications. In a related presentation, Dr. Chelala shared findings from her recent Nature Communications publication, “The Clinical and Molecular Landscape of Breast Cancer in Women of African and South Asian Ancestry,” which revealed that non-European breast cancer patients are often diagnosed at a younger age and face higher mortality rates.
In her presentation “Pregnancy Disorders and Ethnic Diversity: Returning Results,” Professor Catherine Williamson highlighted that pregnancy disorders (such as gestational diabetes, pre-eclampsia and pre-term birth) disproportionately affect Black and ethnic minority women. She also reiterated the statistic that Black women are three times more likely to die from pregnancy-related complications. In a related presentation, Dr. Chelala shared findings from her recent Nature Communications publication, “The Clinical and Molecular Landscape of Breast Cancer in Women of African and South Asian Ancestry,” which revealed that non-European breast cancer patients are often diagnosed at a younger age and face higher mortality rates.
For certain conditions, genomic testing is now proving more effective than traditional methods like ultrasound. However, a lack of data from non-European women, particularly in pre-term birth, has limited progress. In response, Tommy’s National Centre for Preterm Birth Research is actively working to diversify participant representation and engage Black and ethnic minority women in its studies.
Similarly, the Mosaic Community Trust empowers socially and ethnically marginalised communities through educational workshops that help participants understand health, clinical trials and the importance of their involvement in research. Encouragingly, feedback from recent workshops showed a significant increase in the number of individuals willing to participate in future research studies.
Finally, as AI technology becomes increasingly integrated into healthcare, particularly for disease prediction, it is receiving growing attention. This was highlighted at the summit by Dr. Nicole Mathers and colleagues in their discussion “Know Your Data: Applying AI to Bioinformatics,” and at the OSSD Annual Meeting 2025 by Dr. Delisa Fairweather and Dr. Aninye in their session “Big Data and AI in Sex and Gender Research.” A key challenge that must be addressed is the issue of bias in AI systems. AI models learn from the data they are trained on, so if that data is predominantly from white populations, the resulting predictions and insights will reflect those biases. To ensure equitable healthcare, AI must be trained on diverse datasets and designed to actively unlearn ingrained biases so that Black and ethnic minority communities are fairly represented and considered in healthcare decisions.
The Genomics England Health Summit was a powerful reminder of the momentum building in this field—and the importance of collaboration across sectors. We are grateful to everyone who shared their insights and look forward to continuing the conversation.
If you attended the summit and have ideas or feedback, we would love to hear from you!
Follow the Topic
-
Hereditas

Hereditas publishes original cutting-edge research and reviews. The journal welcomes research from across all areas of human, plant, animal and microbial genetics and epigenetics. Topics of interest also include cancer genetics, cancer biology, non-coding RNA, Data Mining, and Genome Technology.
-
Molecular Cytogenetics

Molecular Cytogenetics encompasses all aspects of chromosome biology and the application of molecular cytogenetic techniques in all areas of biology and medicine.
-
Biology of Sex Differences

This journal is unlike any other scientific journal: articles focus on sex differences in all aspects of an individual or organism. Everything from molecules to behavior and from studies of cellular function to clinical research studies are reported in this journal.
Related Collections
With Collections, you can get published faster and increase your visibility.
Optical Genome Mapping (OGM) in Deciphering Genetic Diseases: Advances Towards Clinical Integration
This Collection focuses on the application of Optical Genome Mapping (OGM) technology in the clinical diagnosis of genetic diseases. With its whole - genome molecular markers and high resolution, OGM technology can efficiently detect chromosomal structural variations and easily locate and map the breakpoints or connection points of complex rearrangements. This further explains the functional effects driven by these structural changes, providing new possibilities for the precise diagnosis of genetic diseases.
The Collection will explore the technological advantages of OGM in clinical practice and its continuous innovation, including its high resolution and accuracy in detecting complex genomic variations. It will also discuss how continuous technological innovation can promote the widespread application of OGM in clinical diagnosis, with the expectation of bringing greater clinical benefits to patients with genetic diseases.
We sincerely invite submissions of original research, reviews, and technical reports on OGM technology optimization, methodological validation, and clinical translation studies. Through this Collection, we hope to gather the latest research findings in this field, promote academic exchange, and promote the standardized application of OGM technology in the diagnosis of genetic diseases.
All submissions in this collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Mar 16, 2026
Sex Differences in Metabolic Regulation and End-Organ Damage
Sex differences profoundly influence endocrine and metabolic regulation, shaping disease susceptibility, progression, and therapeutic responses. This Collection aims to highlight how hormonal and metabolic interactions contribute to sex-specific pathophysiology across a range of endocrine disorders and associated systemic complications.
Research topics of interest include mechanisms and therapies addressing hormonal and metabolic dysregulation observed in conditions such as polycystic ovary syndrome (PCOS), metabolic syndrome, diabetic nephropathy, thyroid disorders, Cushing’s syndrome, and hyperaldosteronism. Emphasis is also placed on how these disorders impact multiple organ systems including the cardiovascular and renal systems, resulting in sex-specific patterns of end-organ injury and disease progression. Original research, clinical trials, translational studies, and comprehensive reviews are welcome.
Subtopics include, but are not limited to:
- Hormonal regulation of glucose and lipid metabolism by estrogens, androgens, and progesterone
- Sex-specific metabolic adaptations in obesity, diabetes, and cardiovascular disease
- Neuroendocrine and inflammatory mechanisms linking hormones to metabolic dysregulation
- Cardiovascular and renal complications of endocrine dysfunction
- Neuroendocrine control of metabolic homeostasis
- Endocrine disorders with metabolic manifestations
- Sex-based differences in drug response and therapeutic outcomes
All submissions in this Collection undergo the journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Nov 04, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in