Regaining functionality - how do amputees’ brains incorporate sensory stimulation and combine somatosensory and visual inputs?
Published in Bioengineering & Biotechnology

You are sitting at your desk. You see your phone screen light up and reach out a hand to grab it. Without active thinking, humans naturally use multiple sensory inputs (such as visual and somatosensory) to complete this action. However, the somatosensory pathway is disrupted in individuals with sensorimotor deficits such as limb amputations. Consequently, their ability to perceive somatosensory sensations is lost, and controlling a prosthetic device for object interaction relies primarily on vision.
A sensory neuroprosthesis uses stimulation to provide somatosensory information to individuals with limb amputations. Recent studies show promising results in restoring touch-like sensations and improving prosthesis performance [1-3]. Furthermore, multisensory stimulation paradigms with combined visual and somatosensory inputs showed cognitive benefits such as improved prosthesis perception and control [4-5]. This exciting evidence sheds light on how a sensory neuroprosthesis can help improve the quality of life for individuals with limb amputations.
How does this translate into cortical activity? In particular, when they receive sensory stimulation and pair the sensation with certain hand grips, does their cortical activity show evidence of these combined sensory inputs? Understanding cortical responses is important because this knowledge can inform us how reinstated somatosensory percepts interact with other sensory inputs. To design a truly effective sensory stimulation paradigm, one needs to make sure that the brain is integrating this information effectively.
The study
In our article published in Scientific Reports [link], we sought to answer questions about the influence of sensory stimulation via targeted transcutaneous electrical nerve stimulation (tTENS) on multisensory combination at the cortical level (Figure 1). We worked with three individuals with upper limb amputation from different causes. For each participant, we first found the stimulation location on their residual limb that reliably elicited somatosensory percepts in their phantom hand. We then asked each participant to associate the phantom hand sensation with a hand movement (Figure 1B). For example, one participant linked thumb sensation with hand open. We recorded electroencephalographic (EEG) signals while each participant performed phantom hand movements, presented through a visual cue, with and without tTENS (Figure 1A).
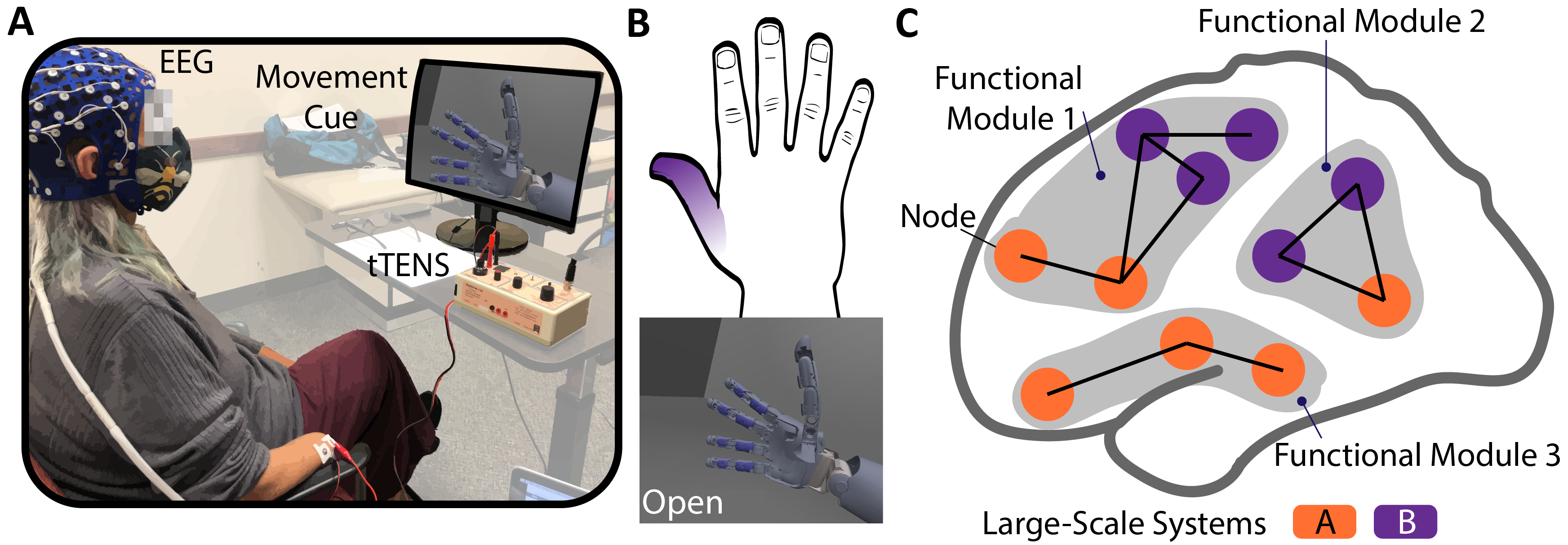
Figure 1. (A) Experimental setup. (B) xample of phantom hand sensation and grasp association identified by one participant. (C) Schematic of the large-scale systems and the functional modules.
The model
We aimed to show how sensorimotor function (i.e., phantom hand grasps performed by our participants) occurs in the cortex. We were interested in the interaction between cortical regions responsible for sensorimotor, visual, and task execution functions. Therefore, we modeled the brain activity (recorded using EEG) as functional networks. This is a graph theory-based method that is able to reveal how activity at different cortical regions is functionally intertwined to achieve motor function and high-level sensorimotor percepts. In particular, we investigated the influence of combined somatosensory and visual inputs on the functional networks.
Our focus was to reveal this functionality through the interaction between large-scale systems via transient functional modules (Figure 1C). Cortical large-scale systems are pre-established systems based on functional engagement (e.g. somatomotor network, default mode network, etc.), while functional modules are formed by parts of the large-scale systems interacting in a transient manner. For example, as a large-scale system, the somatomotor network includes the somatosensory and motor regions in the cortex and is primarily responsible for sensorimotor tasks. The functional modules, rather than being pre-defined based on the literature, were found based on our recorded EEG data during tasks. Based on this modeling, we then carried on to investigate how tTENS changed the interconnections between regions.
Our findings
We found that experience with sensory stimulation matters. The more experience an individual has with tTENS, the more changes in the brain’s modular architecture are observed. Looking at interconnections between large-scale systems, we find enhanced interaction between networks directly associated with a sensory modality (somatomotor and visual networks). For the less tTENS-experienced individuals, we find that higher-level processing, such as the attention network, is more involved. This finding possibly suggests that for the less-experienced participants, more cognitive effort is required to process the sensory stimuli and perform the task.
Significance
Our work demonstrates that the combination of somatosensory and visual inputs is manifested in specific interactions between functional networks in the cortex. These interactions enable efficient integration of cortical information to achieve optimal sensorimotor perception. Insights into how well a stimulation approach promotes high interaction of somatosensory and visual networks are crucial for designing effective stimulation strategies for improved prosthesis perception and control.
References
- Bensmaia, S. J., Tyler, D. J. & Micera, S. Restoration of sensory information via bionic hands. Nat. Biomed. Eng. 7, 1–13 (2020). doi:10.1038/s41551-020-00630-8.
- Raspopovic, S., Valle, G. & Petrini, F. M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 20, 925–939 (2021). doi: 10.1038/s41563-021-00966-9.
- Gonzalez, M., Bismuth, A., Lee, C., Chestek, C. A. & Gates, D. H. Artificial referred sensation in upper and lower limb prosthesis users: a systematic review. J. Neural Eng. 19, 051001 (2022). doi: 10.1088/1741-2552/ac8c38.
- Risso, G. et al. Optimal integration of intraneural somatosensory feedback with visual information: a single-case study. Sci Rep 9, 7916 (2019). doi: 10.1038/s41598-019-43815-1.
- Risso, G. et al. Multisensory stimulation decreases phantom limb distortions and is optimally integrated. iScience 25, 104129 (2022). doi: 10.1016/j.isci.2022.104129.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in