Regulating the Coordination Environment of H2O in Hydrogel Electrolyte for a High-Environment-Adaptable and High-Stability Flexible Zn Devices
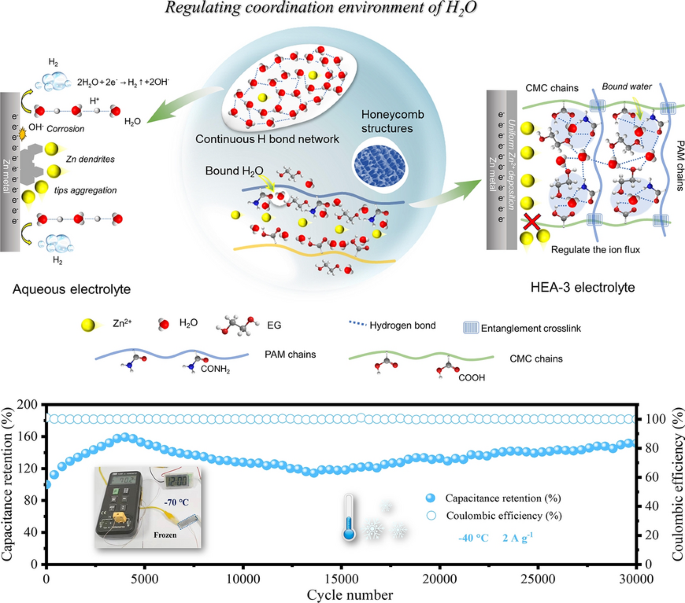
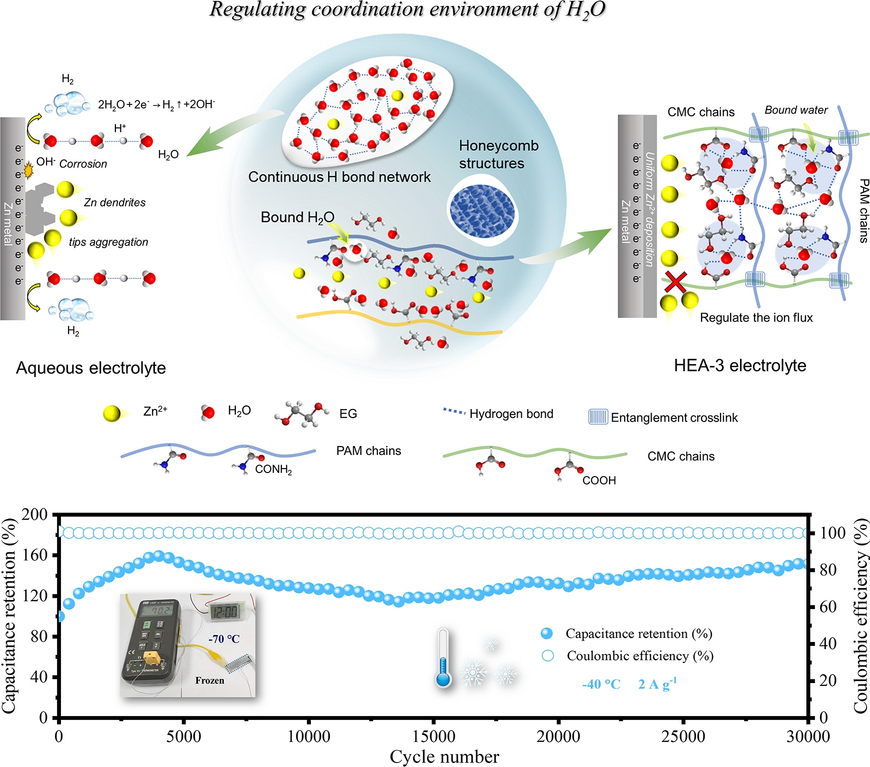
From frigid polar nights to sweltering desert days, powering flexible electronics in extreme environments remains a daunting challenge. Aqueous zinc-ion batteries have long been considered promising for safe, low-cost, and sustainable energy storage, yet they still struggle with zinc dendrite growth, hydrogen evolution, corrosion, and severe performance loss at low temperatures. Now, a research team led by scientists from the Harbin Institute of Technology, the University of Adelaide, and Jiangmen Laboratory of Carbon Science and Technology has unveiled a breakthrough: a high-environment-adaptable hydrogel electrolyte (HEA-3) that precisely regulates the coordination environment of water molecules. Published in Nano-Micro Letters, this work—led by Prof. Zhixin Tai and Prof. Yajie Liu—demonstrates a new pathway for creating flexible zinc-based devices that operate reliably from room temperature down to –70 °C.
Why HEA-3 Matters
- Extreme Temperature Tolerance: Maintains high ionic conductivity (4.12 × 10-3 S cm-1) at –50 °C, with stable cycling even at –70 °C.
- Exceptional Reversibility: Zn||Cu cells achieve 99.4% coulombic efficiency over 900 cycles; Zn||Zn symmetric cells run for over 1,700 hours without dendritic failure.
- Long-Life Flexibility: Flexible Zn||PANI devices deliver more than 30,000 cycles at –40 °C, retaining capacity under folding, twisting, and freezing conditions.
Innovative Design and Mechanisms
- Hydrogel Coordination Engineering: A cross-linked polymer matrix of polyacrylamide (PAM), carboxymethyl cellulose (CMC), and ethylene glycol (EG) disrupts continuous hydrogen-bond networks among water molecules. This reduces proton mobility, suppresses hydrogen evolution, and prevents zinc corrosion.
- Molecular-Level Optimization: DFT and molecular dynamics simulations confirm water molecules preferentially bind with EG, CMC, and PAM rather than with each other, stabilizing a reconstructed multi-hydrogen-bond network.
- Structural Strength and Ionic Transport: The semi-interpenetrating network enhances mechanical durability while interconnected pore channels facilitate uniform Zn2+ transport, yielding a high Zn2+ transference number (0.89) and smooth, dendrite-free zinc deposition.
Applications and Future Outlook
- Wearable Energy Storage: The flexible Zn||PANI device operates under extreme bending and twisting, maintaining over 85% capacity after 1,000 bending cycles—ideal for smart textiles, portable sensors, and medical wearables.
- Cold-Climate Electronics: Stable performance at ultra-low temperatures opens doors for aerospace, polar research, and defense applications.
- Grid-Scale Storage: By mitigating dendrites and corrosion across a wide temperature range, HEA-3 electrolytes could boost the safety and lifespan of large-scale zinc-based energy storage systems.
Looking ahead, the team envisions expanding this water coordination regulation strategy to other metal-ion systems, exploring new polymer frameworks and stimuli-responsive functionalities. This work marks a decisive step toward creating flexible, high-stability, all-weather energy storage devices ready to perform where conventional batteries cannot.
Stay tuned for further breakthroughs as the researchers continue to redefine the limits of aqueous zinc battery technology.
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in