Rethinking Colorectal Cancer Through the Gut Microbiome
Published in Biomedical Research
Colorectal cancer is one of the most intensively studied malignancies, yet it remains remarkably unpredictable. Patients with similar tumours, similar mutations, and similar treatments often follow very different clinical paths. This disconnect was the starting point for our review, recently published in npj Biofilms and Microbiomes, and the reason we began to look beyond the tumour itself 1.
As we explored the literature, one factor kept resurfacing with increasing clarity: the gut microbiome. While microbial associations with colorectal cancer have long been recognized, recent evidence shows that gut microbes are not passive bystanders. They can damage DNA, disrupt epithelial barriers, reshape metabolism, and suppress immune responses. Some bacterial metabolites even leave detectable imprints in human cancer genomes, directly linking microbial exposure to tumour evolution. At that point, treating the microbiome as a secondary or contextual variable no longer made sense 1.
Another realization quickly followed. A single microorganism rarely drives colorectal cancer. Instead, it often involves structured microbial communities organized as biofilms along the intestinal mucosa. Within these communities, different bacteria cooperate metabolically, evade immune clearance, and sustain chronic inflammation. From this perspective, colorectal cancer is best understood not as a disease driven by isolated agents, but as a microbiome-driven ecosystem disease 1.
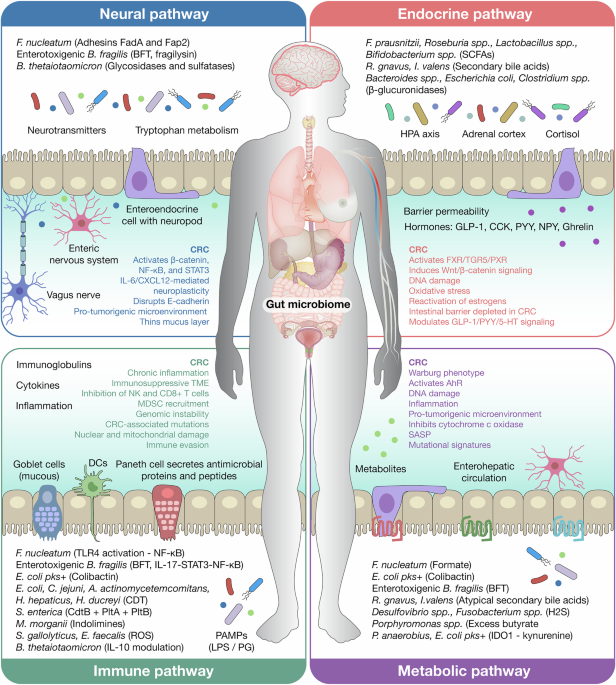
As the review evolved, it became clear that microbial influence on colorectal cancer does not act through a single pathway, but through the coordinated reprogramming of multiple host systems at once. Dysbiotic microbial communities reshape immune composition and function, sustaining inflammation while suppressing effective antitumour responses. In parallel, they alter the metabolic landscape of the tumour microenvironment by modifying nutrient availability and generating bioactive metabolites that fuel tumour growth and impair immune cell fitness 2.
These effects extend beyond local tumour biology. Microbial modulation of endocrine and circadian-regulated signals influences epithelial turnover, inflammatory tone, and immune responsiveness, linking colorectal cancer progression to whole-body physiology 3. At the same time, interactions between microbes and the enteric nervous system integrate microbial cues with autonomic and stress-related signalling, influencing barrier integrity, immune tone, and tumour progression. Together, immune, metabolic, endocrine, and neural pathways form reinforcing feedback loops that stabilize tumour-permissive states and help explain why targeting tumour cells alone is often insufficient 4.
This systems-level view aligns closely with the theme of World Cancer Day, United by Unique. Colorectal cancer is unique not only because tumours differ genetically, but because each patient carries a distinct microbial ecosystem that interacts with their immune system, metabolism, nervous system, and daily rhythms. Cancer emerges from this interaction, not from malignant cells in isolation.
We wrote this review to move the field from association to causation and from description to action. If the microbiome helps drive colorectal cancer, it also becomes a tractable target for prevention and therapy. Approaches such as dietary modulation, microbiota-based interventions, and ecosystem repair strategies begin to make sense when the disease is viewed through this ecological lens 5.
On World Cancer Day, being United by Unique means recognizing that cancer biology is shaped by the systems that make each host different. In colorectal cancer, the gut microbiome is one of the most powerful of those systems. At Universidad de Las Américas (UDLA), Quito-Ecuador, we are now pursuing integrated, systems-level analyses that combine microbial, immune, metabolic, neural, and clinical data to translate this complexity into improved therapeutic strategies and more precise cancer care.
References
- Bautista, J. et al. Gut microbiome-driven colorectal cancer via immune, metabolic, neural, and endocrine axes reprogramming. npj Biofilms and Microbiomes (2026) doi:10.1038/s41522-025-00883-8.
- Bautista, J., Maldonado-Noboa, I., Maldonado-Guerrero, D., Reinoso- Quinga, L. & López-Cortés, A. Microbiome Influence in Gastric Cancer Progression and Therapeutic Strategies. Frontiers in Medicine (2025).
- Bautista, J. & López-Cortés, A. Chronobiome Medicine: Circadian Regulation of Host–Microbiota Crosstalk in Systemic Physiology. Frontiers in Endocrinology (2025).
- Bautista, J. et al. Neurodegeneration rewires the tumor microenvironment via the neuro-immune-cancer axis. iScience 28, 113550 (2025).
- Bautista, J. et al. The Human Microbiome in Clinical Translation: From Bench to Bedside. Frontiers in Microbiology (2025).
Follow the Topic
-
npj Biofilms and Microbiomes

The aim of this journal is to serve as a comprehensive platform to promote biofilms and microbiomes research across a wide spectrum of scientific disciplines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Natural bioactives, Gut microbiome, and human metabolism
Publishing Model: Open Access
Deadline: May 20, 2026
Harnessing plant microbiomes to improve performance and mechanistic understanding
Publishing Model: Open Access
Deadline: Jun 01, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in