Unraveling Druggable Cancer-Driving Proteins Using Artificial Intelligence and Multi-Omics Approaches
Published in Cancer
Cancer remains a major global health challenge, driven by complex biological processes involving numerous proteins. Identifying druggable targets within the cancer proteome is essential for developing precise treatments. Recent advancements in artificial intelligence (AI) and multi-omics analyses have revolutionized our ability to predict druggable proteins, which are proteins capable of binding to small molecules or antibodies to induce clinical effects. This Behind of Paper discusses a novel AI-based approach to predicting druggable cancer-driving proteins and their targeted drugs, paving the way for new therapeutic strategies.
Druggability and the Human Proteome
The human genome contains approximately 19,890 protein-coding genes, but only a small subset is druggable (1–3). Druggability refers to the ability of a protein to bind effectively to a drug-like molecule and induce a therapeutic response (4). Predicting which proteins are druggable is critical for drug discovery, as many promising drug targets fail in clinical development due to lack of druggability. Traditional methods often rely on trial-and-error processes in the lab, but AI and multi-omics approaches provide a more systematic way to identify these targets.
AI and Machine Learning in Predicting Druggable Proteins
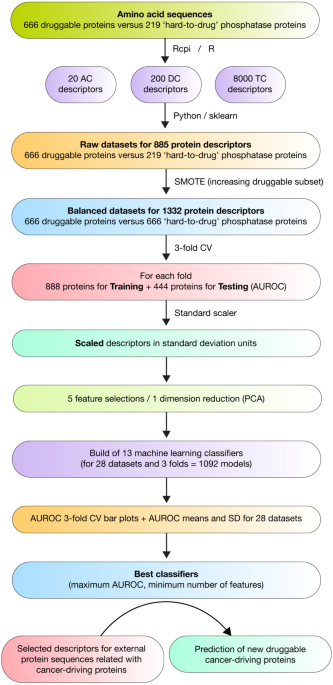
By leveraging AI, we have developed classifiers that predict druggable cancer-driving proteins based on their amino acid composition. In this study, 13 different machine learning classifiers were trained on amino acid sequence descriptors to identify druggable proteins. The support vector machine (SVM) model, using 200 tri-amino acid composition descriptors, emerged as the best-performing classifier with an impressive area under the receiver operating characteristic (AUROC) of 0.975 (5,6).
These predictions were further validated using multi-omics approaches, including ligandability assessment, prognostic protein analysis, and drug repurposing studies. Multi-omics data provides comprehensive insights into protein interactions, mutations, and expression patterns, helping to fine-tune the predictive models. In this study, 79 druggable cancer-driving proteins were identified, and 23 of them were linked to unfavorable prognoses in several cancer types. These include notable proteins such as CDKN2A, BCL10, ACVR1, CASP8, JAG1, TSC1, NBN, PREX2, PPP2R1A, DNM2, VAV1, ASXL1, TPR, HRAS, BUB1B, ATG7, MARK3, SETD2, CCNE1, MUTYH, CDKN2C, RB1, and SMARCA4 (5,6).
Drug Repurposing and Clinical Implications
Drug repurposing is another significant outcome of our study. This strategy reduces the time and cost of developing new therapies by using already-approved drugs in new contexts, such as treating different cancers. By evaluating existing drugs for their potential to target predicted druggable proteins, we have identified 11 clinically relevant drugs: mifepristone, pentostatin, afatinib, alitretinoin, talazoparib, alpelisib, ulipristal acetate, lorlatinib, piflufolastat, pyrvinium pamoate, and tepotinib hydrochloride.
Mifepristone, a progesterone receptor antagonist, has been explored for its potential in treating glioblastoma, breast cancer, and uveal melanoma due to its ability to act on multiple receptor types, including glucocorticoid and androgen receptors (7–9). Pentostatin is a chemotherapy drug primarily used for treating hairy cell leukemia and T-cell prolymphocytic leukemia. It is a purine analog that works by inhibiting the enzyme adenosine deaminase, crucial for DNA synthesis and cell replication, leading to the accumulation of deoxyadenosine triphosphate and ultimately causing cell death, particularly in rapidly dividing cancer (10). Afatinib is an oral medication primarily used for treating non-small cell lung cancer. It functions as a tyrosine kinase inhibitor, targeting and blocking the EGFR protein as well as other members of the ErbB family, including HER2 and ErbB4 (11). Alitretinoin, a derivative of vitamin A, is used in cancer treatment primarily for Kaposi sarcoma. It binds to and activates retinoid receptors (RAR and RXR), which regulate gene expression involved in cell differentiation and proliferation, helping to inhibit the growth of Kaposi sarcoma cells (12). Talazoparib works by inhibiting PARP enzymes, which play a crucial role in DNA repair. By blocking these enzymes, talazoparib prevents cancer cells from repairing their DNA, leading to cell death, especially in cells with BRCA1/2 mutations that already have compromised DNA repair mechanisms (13,14). Alpelisib is an oral medication used in combination with fulvestrant to treat hormone receptor-positive, HER2-negative advanced or metastatic breast cancer with PIK3CA mutations. It works as a PI3K inhibitor, specifically targeting the alpha isoform of the enzyme, which is crucial in the PI3K/AKT signaling pathway involved in cancer cell growth and survival (15). Ulipristal acetate is a progesterone receptor modulator implicated in the proliferation and growth of certain cancer cells. It competes with progesterone, thereby inhibiting the progesterone-induced proliferation of breast cancer cells, making it a candidate for reducing breast cancer risk, especially in individuals with BRCA1/2 mutations (16). Lorlatinib inhibits ALK and ROS1 kinases, which are involved in cancer cell growth and survival. It is effective against multiple ALK mutations that confer resistance to first- and second-generation ALK inhibitors (17). Piflufolastat F-18 binds to the prostate-specific membrane antigen, a protein overexpressed on the surface of most prostate cancer cells. Once bound, the radioactive tracer emits positrons detected by a PET scanner, revealing the location of PSMA-positive lesions in the body (18). Pyrvinium pamoate is an androgen receptor antagonist that targets multiple cellular pathways. It disrupts mitochondrial function by inhibiting electron transport chain complexes I and II, reducing mitochondrial fitness and increasing glycolysis, especially under hypoglycemic conditions often found in tumors. It also reduces WNT and Hedgehog signaling pathways, crucial for cancer cell proliferation and survival (19–22). Lastly, tepotinib hydrochloride is a tyrosine kinase inhibitor targeting the MET receptor. By inhibiting this receptor, it interferes with cancer cell growth and survival pathways, which are crucial for the proliferation and metastasis of MET-altered cancer cells.
Conclusion
The integration of AI, machine learning, and multi-omics approaches has proven effective in predicting druggable cancer-driving proteins and identifying potential therapeutic targets. These methods accelerate the path to precision oncology and personalized cancer therapies by prioritizing key proteins and repurposing existing drugs. As these computational models continue to evolve, they will play a critical role in overcoming the challenges of drug discovery and improving cancer patient outcomes.
References
- Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, et al. The complete sequence of a human genome. Science. 2022 Apr;376(6588):44–53.
- Barbarino JM, Whirl-Carrillo M, Altman RB, Klein TE. PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417.
- Venter JC, Smith HO, Adams MD. The sequence of the human genome. Clin Chem. 2015 Sep;61(9):1207–8.
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015 Jan 23;347(6220):1260419.
- López-Cortés A, Cabrera-Andrade A, Echeverría-Garcés G, Echeverría-Espinoza P, Pineda-Albán M, Elsitdie N, et al. Unraveling druggable cancer-driving proteins and targeted drugs using artificial intelligence and multi-omics analyses. Sci Rep. 2024 Aug 21;14(1):19359.
- López-Cortés A, Cabrera-Andrade A, Vázquez-Naya JM, Pazos A, Gonzáles-Díaz H et al. Prediction of breast cancer proteins involved in immunotherapy, metastasis, and RNA-binding using molecular descriptors and artificial neural networks. Sci Rep. 2020 May 22;10(1):8515.
- Llaguno-Munive M, Vazquez-Lopez MI, Jurado R, Garcia-Lopez P. Mifepristone Repurposing in Treatment of High-Grade Gliomas. Front Oncol. 2021 Feb 18;11:606907.
- Alvarez PB, Laskaris A, Goyeneche AA, Chen Y, Telleria CM, Burnier JV. Anticancer effects of mifepristone on human uveal melanoma cells. Cancer Cell Int. 2021 Nov 17;21(1):607.
- Elía A, Saldain L, Vanzulli SI, Helguero LA, Lamb CA, Fabris V, et al. Beneficial Effects of Mifepristone Treatment in Patients with Breast Cancer Selected by the Progesterone Receptor Isoform Ratio: Results from the MIPRA Trial. Clin Cancer Res. 2023 Mar 1;29(5):866–77.
- Cassileth PA, Cheuvart B, Spiers AS, Harrington DP, Cummings FJ, Neiman RS, et al. Pentostatin induces durable remissions in hairy cell leukemia. J Clin Oncol. 1991 Feb;9(2):243–6.
- Harada Y, Sato A, Nakamura H, Kai K, Kitamura S, Nakamura T, et al. Anti-cancer effect of afatinib, dual inhibitor of HER2 and EGFR, on novel mutation HER2 E401G in models of patient-derived cancer. BMC Cancer. 2023 Jan 23;23(1):77.
- Htet KZ, Waul MA, Leslie KS. Topical treatments for Kaposi sarcoma: A systematic review. Skin Health and Disease. 2022 Jun;2(2):e107.
- Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med. 2018 Aug 23;379(8):753–63.
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074–82.
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019 May 16;380(20):1929–40.
- Bartlett TE, Evans I, Jones A, Barrett JE, Haran S, Reisel D, et al. Antiprogestins reduce epigenetic field cancerization in breast tissue of young healthy women. Genome Med. 2022 Jun 15;14(1):64.
- Kumar A, Kapoor A, Noronha V, Patil V, Menon N, Singh AK, et al. Lorlatinib in the second line and beyond for ALK positive lung cancer: real-world data from resource-constrained settings. BJC Rep. 2024 May 1;2(1):35.
- Arafa AT, Jain A, Skrobanek P, Humphrey B, Froelich JW, Antonarakis ES. Impact of piflufolastat F-18 PSMA PET imaging on clinical decision-making in prostate cancer across disease states: A retrospective review. Prostate. 2023 Jun;83(9):863–70.
- Ponzini FM, Schultz CW, Leiby BE, Cannaday S, Yeo T, Posey J, et al. Repurposing the FDA-approved anthelmintic pyrvinium pamoate for pancreatic cancer treatment: study protocol for a phase I clinical trial in early-stage pancreatic ductal adenocarcinoma. BMJ Open. 2023 Oct 17;13(10):e073839.
- Tomitsuka E, Kita K, Esumi H. An anticancer agent, pyrvinium pamoate inhibits the NADH-fumarate reductase system--a unique mitochondrial energy metabolism in tumour microenvironments. J Biochem. 2012 Aug;152(2):171–83.
- Ishii I, Harada Y, Kasahara T. Reprofiling a classical anthelmintic, pyrvinium pamoate, as an anti-cancer drug targeting mitochondrial respiration. Front Oncol. 2012 Oct 2;2:137.
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Related Collections
With Collections, you can get published faster and increase your visibility.
Obesity
Publishing Model: Hybrid
Deadline: Apr 24, 2026
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in