
It is crucial for the innate immune system to detect microbes and more precisely to sense pathogens. One strategy is the detection of virulence factors that are specifically expressed by pathogenic microbes 1. Numerous pathogenic bacteria express virulence factors targeting Rho proteins. This feature is certainly related to their involvement in the regulation of the dynamic of the actin cytoskeleton and therefore the innate immune processes such as phagocytosis or leucocyte migration. RhoGTPase-targeting toxins either activates or inactivates RhoGTPases with the same goal of hijacking the host immune response.
The CNF1 toxin, from uropathogenic Escherichia coli (UPEC), is a bona fide RhoGTPase activating toxin. CNF1 is endowed with a deamidase activity that modifies RhoGTPases thereby destroying their intrinsic or GAP-stimulated ability to hydrolyze GTP, locking them in a GTP bound active form with the consequence of RhoGTPases constitutive activation of their downstream signaling pathways. We have shown previously that CNF1 triggers a protective immune response during bacteremia in mice based on the activation of Caspase-1 and secretion of the IL-1ß cytokine, suggesting the potential involvement of an inflammasome 2. Inflammasomes are molecular platforms assembled in response to DAMPs or PAMPs sensing that activate Caspase-1 which processes pro-IL-1ß into biologically active IL-1ß 3.
In our recently published paper, we show that the NLRP3 inflammasome acts as a sensor of the Rac2 GTPase activation triggered by various bacterial toxins and virulence factors. The NLRP3 inflammasome is necessary for the Caspase-1 activation and subsequent IL-1ß secretion/maturation by macrophages in response to the treatment by CNF1 from E. coli, SopE from Salmonella and DNT from Bordetella. We show that CNF1-induced NLRP3 activation depends on the inflammasome regulator Nek7 which acts downstream of K+ efflux. We demonstrate that the Pak1 kinase, a Rac2 effector, is essential for CNF1-induced NLRP3 inflammasome activation. Indeed, activated Pak1 phosphorylates NLRP3 on the Threonine 659 allowing NLRP3-Nek7 interaction and assembly of the inflammasome components. We demonstrate in vivo the signaling axis Pak1-NLRP3 as critical for the bacterial clearance of E. coli expressing CNF1 during bacteremia in mice. Importantly, we show that Pak1-mediated NLRP3 Thr659 phosphorylation is involved in inflammasome activation by other NLRP3 inflammasome triggers such as Nigericin. A major difference compared to well-known NLRP3 activators is that CNF1-triggered IL-1ß secretion is independent of pyroptosis and does not induce Gasdermin D (GSDMD) cleavage and pore formation. However, the mechanism involved in GSDMD-independent IL-1ß secretion in response to RhoGTPases activation remains to be discovered.
Check out our new publication here: https://www.nature.com/articles/s41564-020-00832-5
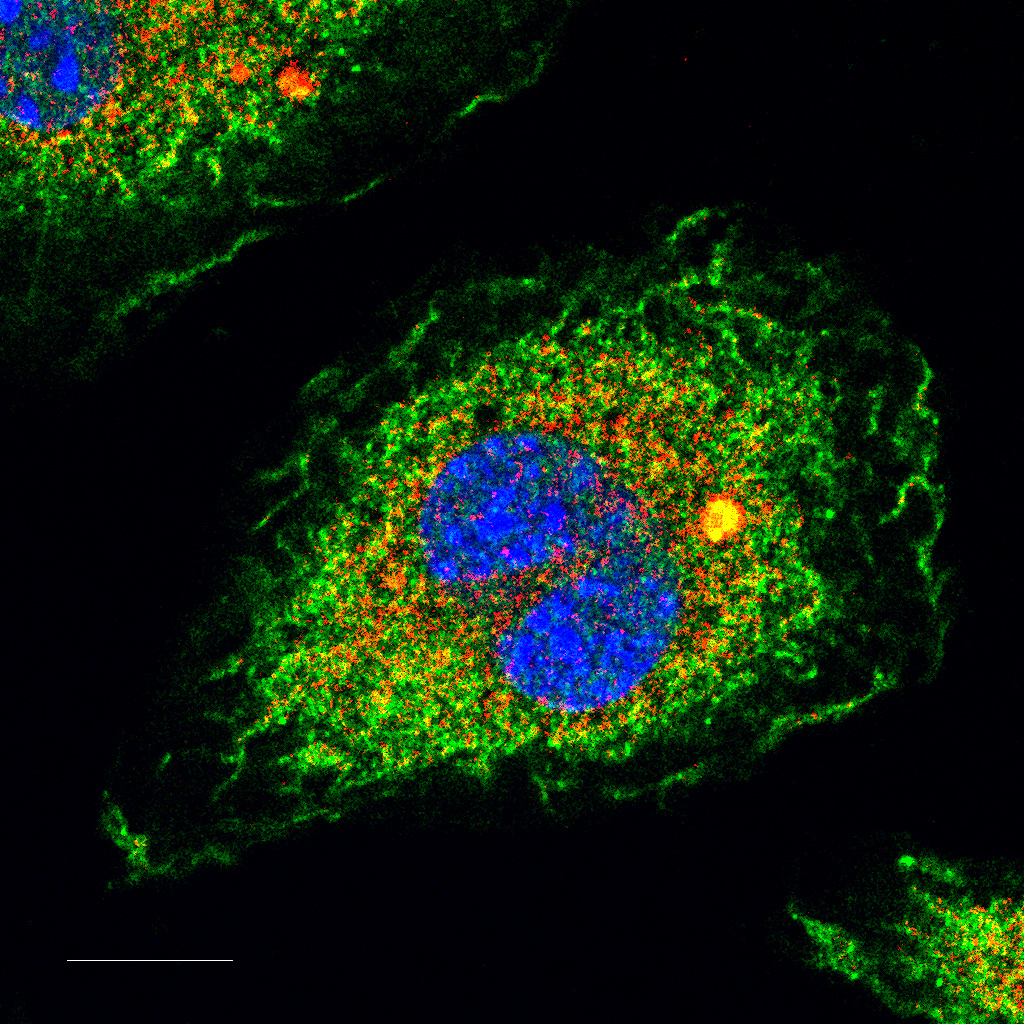
NLRP3 Inflammasome senses RhoGTPases activating toxins. Immunofluorescence staining of bone marrow derived macrophage showing endogenous NLRP3 (red) and Nek7 (green) colocalizing in an inflammasome Speck-like structure upon treatment with the RhoGTPases activating toxin CNF1 from Escherichia coli. Nuclei are in blue. Scale bar: 10 µm
References
- Stuart, L. M., Paquette, N. & Boyer, L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat. Rev. Immunol. 13, 199–206 (2013).
- Diabate, M. et al. Escherichia coli α-hemolysin counteracts the anti-virulence innate immune response triggered by the Rho GTPase activating toxin CNF1 during bacteremia. PLoS Pathog. 11, e1004732 (2015).
- Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in