RNF112 facilitates ubiquitin-mediated degradation of c-Myc, suppressing proliferation, migration and lipid synthesis in bladder cancer
Published in Cancer
The incidence of bladder cancer (BLCA) exceeds 500,000 cases annually and is predicted to double by 2040, according to the World Health Organization1. Notably, one-quarter of newly diagnosed BLCA patients have muscle-invasive BLCA or metastatic BLCA, which severely impacts patient survival2. The 5-year mortality rate for muscle-invasive BLCA patients with lymph node metastasis exceeds 77%3 . Therefore, exploring new mechanisms of BLCA tumorigenesis and metastasis and developing potential targets for treatment are important for improving the prognosis of patients with BLCA.
The ubiquitin-proteasome system is critical for tumors and influences the protein stability of several key molecules associated with tumorigenesis, progression, metastasis, and chemotherapy resistance4. Our previous studies confirmed several E3 ligases that are strongly associated with BLCA proliferation and metastasis5, 6. The RING finger (RNF) protein family comprises important E3 ligases. Our previous study revealed that RNF126 can facilitate the progression of BLCA by targeting the pivotal protein PTEN for degradation6. Subsequent investigations revealed that the expression of RNF112, another member of the RNF protein family, was notably downregulated in BLCA tissues.
c-Myc, a transcription factor, is essential for various biological processes, including cell cycle regulation, metabolic reprogramming, epithelial-mesenchymal transition, and signal transduction7. Our previous studies revealed that several key molecules (e.g., POLD1, USP43 and TRAIP) interact with c-Myc to regulate its ubiquitination and protein stability, thereby affecting BLCA progression5, 8, 9. Lipid metabolism is closely related to the risk, progression, and metastasis of BLCA10. c-Myc is a regulator of various lipid metabolism-related enzymes11, and whether it is involved in the regulation of lipid metabolism in BLCA and the possible underlying mechanisms remain to be further studied.
This study revealed that RNF112 expression was significantly reduced in BLCA. In vitro and in vivo studies indicated that RNF112 suppresses BLCA cell proliferation, migration, and lipid synthesis. Mechanistically, RNF112 directly interacts with the MB II domain of MYC through its N-terminal zinc finger motif, and its catalytic site C97 facilitates K48-linked polyubiquitination of the K389 residue on the c-Myc protein, accelerating its degradation. Additionally, our research validated the interaction of c-Myc with the promoter of ATP citrate lyase (ACLY), a central enzyme of lipid metabolism, promoting its transcriptional activity. The restoration of c-Myc or ACLY expression attenuated the inhibitory effects of RNF112 on BLCA cell growth, migration, and lipid synthesis.
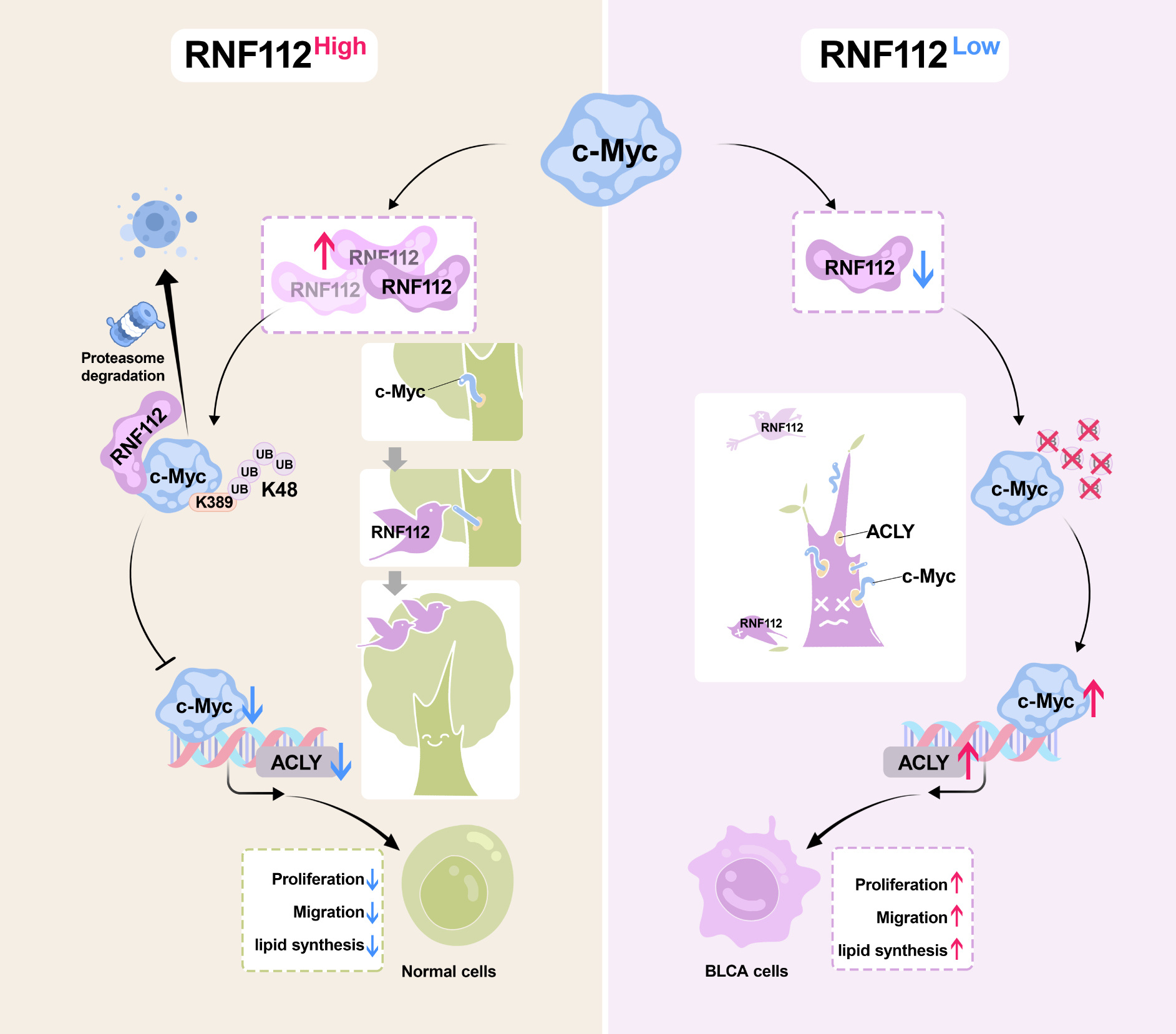
In summary, our study confirmed that RNF112 suppressed the proliferation, migration, and lipid synthesis of BLCA cells by facilitating the ubiquitin-mediated degradation of c-Myc.
Dr. Gang Wang, Dr. Xinghuan Wang, and Dr. Yu Xiao are the corresponding authors of this paper, with MD candidate Kangping Xiong, MD candidate Siming Chen, Dr. Huimin Xu, and MD candidate Sheng Tu serving as co-first authors.
References
- Dyrskjøt, L. et al. Bladder cancer. Nat Rev Dis Primers 9, 58 (2023).
- van Hoogstraten, L.M.C. et al. Global trends in the epidemiology of bladder cancer: challenges for public health and clinical practice. Nat Rev Clin Oncol 20, 287-304 (2023).
- Zhang, R. et al. Suppressive effects of plumbagin on the growth of human bladder cancer cells via PI3K/AKT/mTOR signaling pathways and EMT. Cancer Cell Int 20, 520 (2020).
- Wang, M., Zhang, Z., Li, Z., Zhu, Y. & Xu, C. E3 ubiquitin ligases and deubiquitinases in bladder cancer tumorigenesis and implications for immunotherapies. Front Immunol 14, 1226057 (2023).
- Yu, J. et al. TRAIP suppresses bladder cancer progression by catalyzing K48-linked polyubiquitination of MYC. Oncogene 43, 470-483 (2024).
- Xu, H. et al. E3 ubiquitin ligase RNF126 affects bladder cancer progression through regulation of PTEN stability. Cell Death Dis 12, 239 (2021).
- Das, S.K., Lewis, B.A. & Levens, D. MYC: a complex problem. Trends Cell Biol 33, 235-246 (2023).
- Wang, Y. et al. DNA polymerase POLD1 promotes proliferation and metastasis of bladder cancer by stabilizing MYC. Nature communications 14, 2421 (2023).
- Li, M. et al. USP43 stabilizes c-Myc to promote glycolysis and metastasis in bladder cancer. Cell Death Dis 15, 44 (2024).
- Zhou, L. et al. Metabolic reprogramming based on RNA sequencing of gemcitabine-resistant cells reveals the FASN gene as a therapeutic for bladder cancer. J Transl Med 22, 55 (2024).
- Gouw, A.M. et al. The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab 30, 556-572.e555 (2019).
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in