Screening Anionic Groups Within Zwitterionic Additives for Eliminating Hydrogen Evolution and Dendrites in Aqueous Zinc Ion Batteries
As global demand grows for safe, low-cost, and sustainable energy storage technologies, aqueous zinc-ion batteries (AZIBs) have gained increasing attention due to their high theoretical capacity, environmental friendliness, and intrinsic safety. However, their practical application has long been hindered by two major challenges: uncontrolled zinc dendrite growth and hydrogen evolution reactions (HER), both of which degrade battery performance and lifespan.
Now, a collaborative research team from Nanjing University, The University of Queensland, and Shanghai Jiao Tong University has developed an innovative solution using zwitterionic electrolyte additives. Their findings, published in Nano-Micro Letters, offer a promising pathway toward ultra-stable and long-life AZIBs.
Why This Matters
- Dendrite-Free Zinc Deposition: The additive promotes uniform deposition along the Zn (002) crystal plane, effectively suppressing dendrite formation.
- Hydrogen Evolution Suppression: It stabilizes the local pH environment, significantly reducing HER and associated parasitic reactions.
- Long Cycle Life: The modified electrolyte enables over 4,000 charge–discharge cycles with an ultra-low average capacity decay of just 0.014% per cycle.
Key Innovation: Molecular Design Matters
The researchers systematically compared three zwitterionic compounds—CBMA, SBMA, and MPC—all featuring the same quaternary ammonium cation but different anionic groups: carboxylate, sulfonate, and phosphate, respectively.
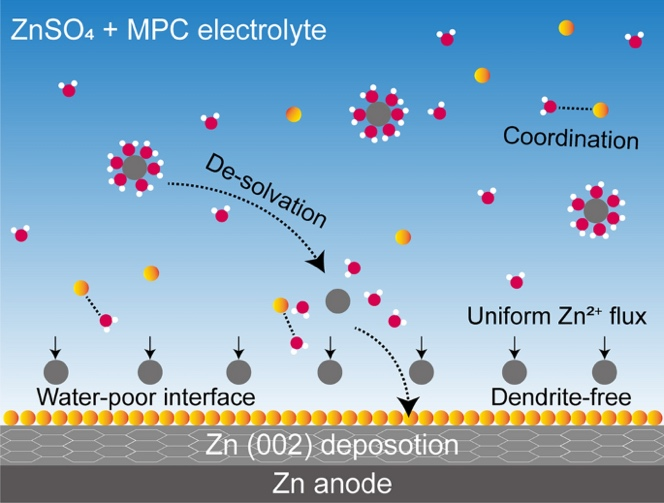
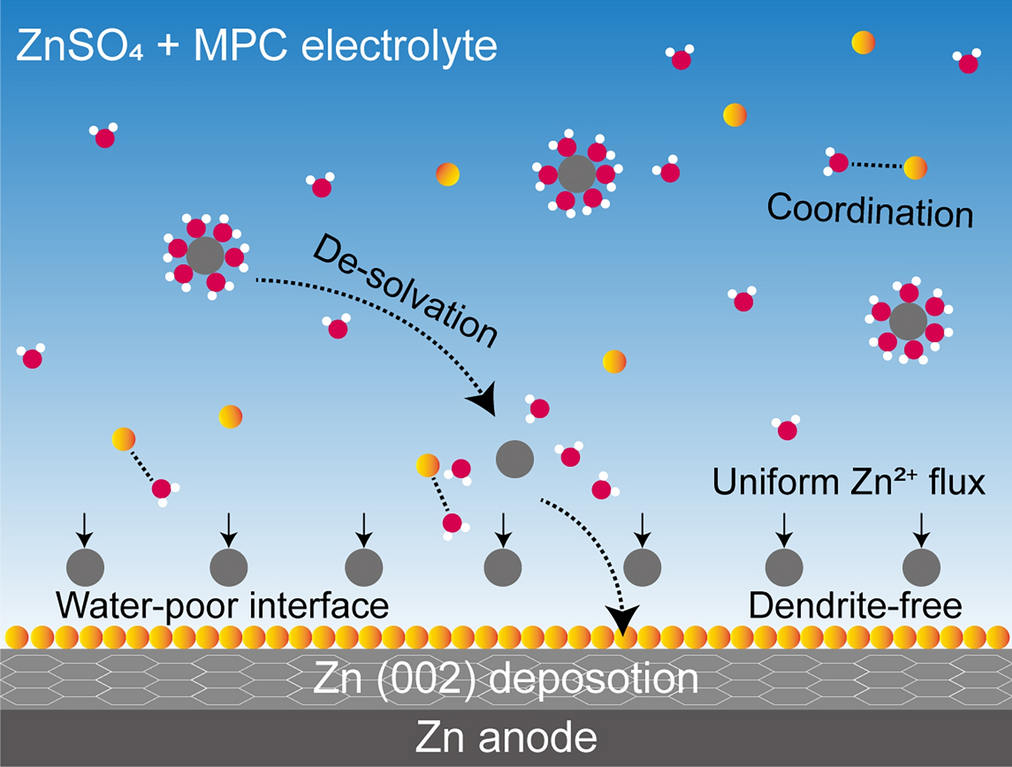
Among them, MPC (2-methacryloyloxyethyl phosphorylcholine) emerged as the most effective additive due to its unique dual functionality:
- pH Buffering: The phosphate group can buffer both H+ and OH- ions, maintaining a stable electrolyte environment during cycling.
- Zn (002) Orientation: Promotes dense, flat zinc deposition, minimizing surface roughness and suppressing dendrite formation.
- Enhanced Desolvation: Reduces the activation energy required for Zn2+ desolvation, mitigating water-induced side reactions and improving plating/stripping reversibility.
Performance Highlights
- Zn//Zn symmetric cell: Stable cycling for over 5,000 hours at 1 mA cm-2 without short-circuiting.
- Zn//Cu half-cell: Achieves a high Coulombic efficiency of 99.6% over 600 cycles.
- Full cell (Zn//NaVO): Demonstrates exceptional stability over 4,000 cycles at 5 A g-1 with only 0.014% capacity fade per cycle.
- Pouch cell prototype: Retains 86.6% of its initial capacity after 300 cycles under high current density and low N/P ratio (~3.6), showing strong practical potential.
Mechanistic Insights
Using a combination of electrochemical analysis, DFT calculations, and molecular dynamics simulations, the team revealed that:
- Zwitterions adsorb preferentially on the Zn surface, forming a protective interfacial layer.
- MPC enhances Zn2+ flux uniformity and guides oriented deposition along the thermodynamically stable (002) plane.
- The additive reconstructs the solvation shell of Zn2+, reducing water activity and suppressing HER.
Future Outlook
This work highlights the critical role of anionic group selection in zwitterionic additives and demonstrates how molecular-level electrolyte engineering can solve long-standing challenges in AZIBs. The compatibility, low cost, and scalability of MPC make it a highly promising candidate for next-generation energy storage systems.
Moreover, the MPC molecule includes a polymerizable methacrylate group, opening the door for future development of functional polymer electrolytes and solid-state batteries.
Stay tuned for more exciting developments from this interdisciplinary research team as they continue to push the boundaries of safe, sustainable, and high-performance energy storage technologies!
Follow the Topic
-
Nano-Micro Letters

Nano-Micro Letters is a peer-reviewed, international, interdisciplinary and open-access journal that focus on science, experiments, engineering, technologies and applications of nano- or microscale structure and system in physics, chemistry, biology, material science, and pharmacy.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in