Sensitizing Acquired Radioresistant Tumours to Re-Irradiation
Published in Bioengineering & Biotechnology, Materials, and Biomedical Research

Acquired radioresistance in Re-Irradiation
In the relentless battle against cancer, radiotherapy has long stood as a formidable weapon, utilizing the power of ionizing radiation to destroy tumour cells. Yet, the specter of acquired radiation resistance haunts this treatment, as it allows malignant cells to shield themselves from the therapeutic effects, leading to recurrence and metastasis, a predicament that often leads to the need for re-irradiation. Re-irradiation, the process of treating a previously irradiated area with ionizing radiation, is complicated by the increased radiation resistance of recurrent tumours. High-dose radiation is required to achieve the desired effect, but this approach is limited by the tolerance of normal tissues to radiation doses, especially when the radiation field overlaps or when the interval between re-irradiations is short. The quest to enhance the effectiveness of radiotherapy has led to the development of precise radiation techniques such as intensity-modulated radiotherapy, volumetric-modulated arc therapy, and brachytherapy. Yet, the development of radiosensitization strategies for tumours with acquired radioresistance remains a formidable challenge.
Cuproptosis as a novel radiosensitizing target
Dr. Zhanjun Gu’s group from Institute of High Energy Physics, Chinese Academy of Science, is dedicated to developing novel and translationable radiosenstizer and radioprotector over the years. Through the in-depth collaboration with Dr. Fuquan Zhang and Dr. Junfang Yan from the Radiotherapy Department of Peking Union Medical College Hospital, we discovered a new radiosensitizing mechanism based on cupropotosis (a copper-dependent form of programmed cell death). Our journey began with the observation that residual tumours, those tenacious survivors of initial radiotherapy, exhibit upregulation of key proteins involved in cuproptosis, namely FDX1 and LIAS (Figure 1). This serendipitous discovery suggested that these resistant cells might harbor an Achilles' heel: an increased susceptibility to cuproptosis. Thus, we set forth to develop a radiosensitizer that could exploit this vulnerability. To test this, we designed and synthesized a copper-containing polyoxometalate, PWCu, as a controllable cuproptosis-targeting sensitizing agent for re-irradiation.
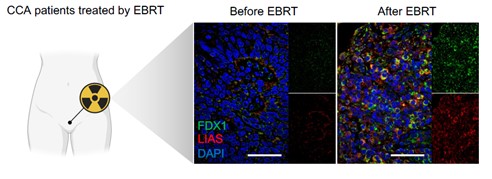 Figure 1. Cuproptosis-related key proteins are up-regulated after EBRT.
Figure 1. Cuproptosis-related key proteins are up-regulated after EBRT.
The PWCu nanocapsules could effectively internalize into cancer cells and, upon irradiation, release copper ions to trigger cuproptosis. A significant increase in cell death demonstrates the ability of PWCu nanocapsules to overcome acquired radioresistance. Moreover, the nanocapsules activated a robust abscopal effect, a phenomenon where irradiating one tumour site leads to the regression of distant, untreated tumours. The mechanism behind this synergistic effect is rooted in the unique interaction between the PWCu nanocapsules and the tumour microenvironment. The release of copper ions leads to the destabilization of Fe-S cluster proteins and the aggregation of lipoylated proteins, hallmarks of cuproptosis. Furthermore, the PWCu nanocapsules could induce the expression of heat shock protein 70 (HSP70), promote the exposure of calreticulin (CRT), and enhance the release of high mobility group box 1 (HMGB1), all of which are key mediators of immunogenic cell death. This suggests that the PWCu nanocapsules offer a multifaceted strategy that not only enhances the local antitumour effects of radiotherapy but also activates systemic antitumour immunity. The potential of this approach to improve patient outcomes, especially in the context of recurrent and metastatic diseases, is immense (Figure 2).
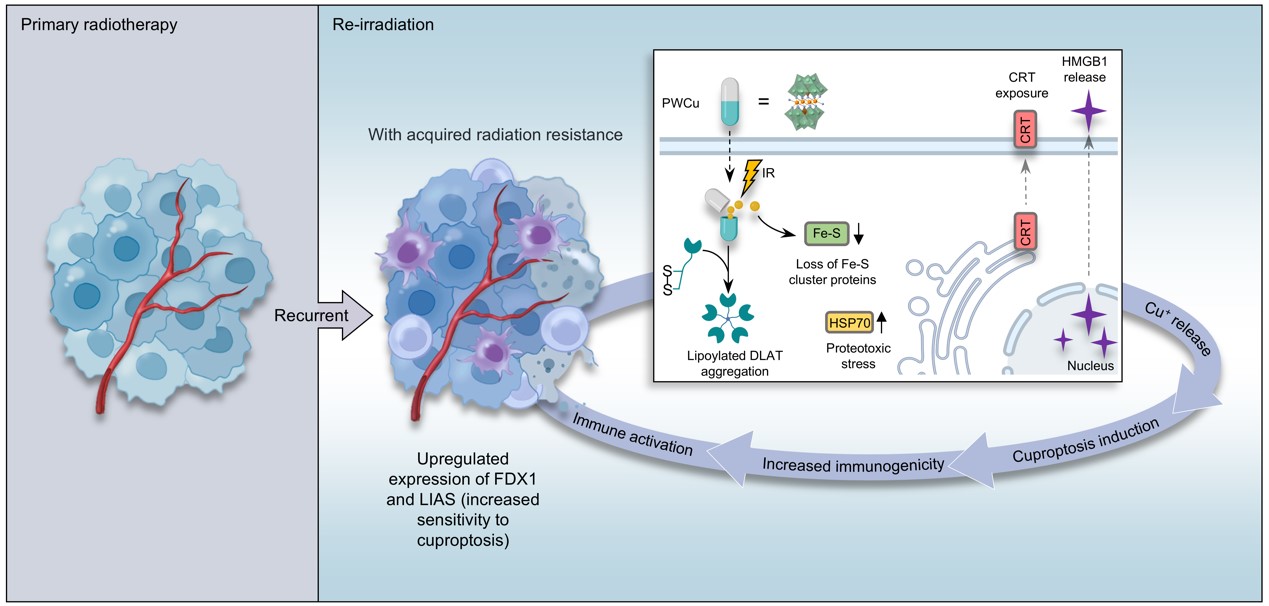
Figure 2. Re-irradiation sensitization mediated by copper-containing nanocapsules.
Where do we go from here?
Future research will center on examining the synergistic impact of combining cuproptosis-targeted radiosensitization with immunotherapy and refining the copper-containing radiosensitizer for clinical use. To advance clinical translation, it is vital to enhance collaboration with physicians and pharmaceutical companies to assess the safety profile of copper drug-based radiosensitizers. Pilot production will also be conducted to meet market needs. Ultimately, our goal is to offer practical solutions for improving radiotherapy outcomes in clinical settings.
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in